Job review #1472
Job #1472 review - http://old.mole.upol.cz/online/1472/
Generated on 2025-02-02 14:00:50 by service v2.13.8.2.
5 tunnels click to expand / contract
-
show | | profile | lining residues
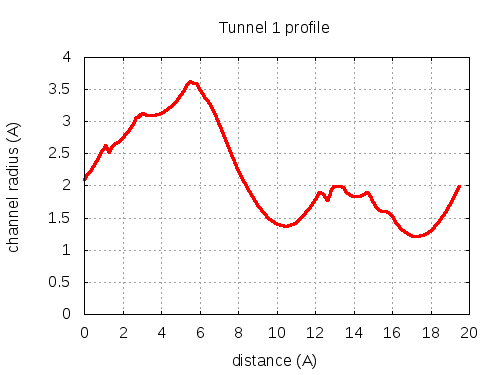
Unique lining residues set - all
73 ALA C, 74 THR C, 75 THR C, 100 ILE C, 103 PHE C, 100 ILE C, 300100 ILE C, 104 GLY C, 107 THR C, 200107 THR C, 103 PHE C, 300101 THR C, 300100 ILE C, 106 VAL C, 110 LEU C, 300101 THR C
Unique lining residues set - sidechains
75 THR C, 103 PHE C, 100 ILE C, 300100 ILE C, 107 THR C, 200107 THR C, 106 VAL C, 110 LEU C, 300101 THR C
Physicochemical properties of lining side-chains
Charge: 0 (0-0)
Hydropathy: 1.4
Hydrophobicity: 0.24
Polarity: 1.55
Mutability: 93
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 73 ALA C, 74 THR C, 75 THR C, 100 ILE C, 103 PHE C 2.11 0.45 0.18 -0.36 2.43 79 2 74 THR C, 75 THR C, 103 PHE C, 100 ILE C 2.3 0.65 1.55 0.4 1.38 87 3 74 THR C, 75 THR C, 100 ILE C, 103 PHE C 2.4 1.88 0.33 -0.26 2.19 79 4 75 THR C, 103 PHE C, 100 ILE C 2.72 2.22 2.2 0.8 0.71 87 5 75 THR C, 103 PHE C, 100 ILE C, 300100 ILE C 2.83 2.71 2.78 1.05 0.57 91 6 75 THR C, 103 PHE C, 100 ILE C 3.06 3 2.2 0.8 0.71 87 7 75 THR C, 103 PHE C, 100 ILE C, 300100 ILE C 3.1 3.87 2.78 1.05 0.57 91 8 103 PHE C, 100 ILE C, 300100 ILE C 3.11 4.48 3.93 1.66 0.2 85 9 103 PHE C, 100 ILE C, 300100 ILE C, 104 GLY C 3.23 5.72 2.85 1.04 1 85 10 100 ILE C, 104 GLY C, 107 THR C 3.6 5.79 1.13 0.08 1.72 105 11 100 ILE C, 104 GLY C, 200107 THR C 3.58 5.97 1.13 0.08 1.72 105 12 100 ILE C, 104 GLY C, 107 THR C 3.49 6.47 1.13 0.08 1.72 105 13 103 PHE C, 300100 ILE C, 104 GLY C, 107 THR C 3.28 7.38 1.55 0.4 1.38 87 14 103 PHE C, 300100 ILE C, 107 THR C 1.41 12 2.2 0.8 0.71 87 15 300100 ILE C, 107 THR C, 103 PHE C 1.82 12.45 1.13 0.08 1.72 105 16 300100 ILE C, 107 THR C, 103 PHE C, 300101 THR C 1.83 12.6 0.75 -0.14 2.14 105 17 107 THR C, 103 PHE C, 300101 THR C, 300100 ILE C 1.78 12.74 -0.48 -0.79 2.95 107 18 300100 ILE C, 107 THR C, 103 PHE C, 300101 THR C 1.9 13.07 0.75 -0.14 2.14 105 19 107 THR C, 103 PHE C, 300101 THR C, 300100 ILE C, 106 VAL C 2 13.26 0.46 -0.41 2.39 102 20 107 THR C, 103 PHE C, 300101 THR C, 106 VAL C 1.85 14.12 0.68 -0.31 2.14 102 21 107 THR C, 300101 THR C, 106 VAL C 1.83 14.42 1.03 -0.15 1.72 102 22 107 THR C, 300101 THR C, 106 VAL C, 110 LEU C 1.75 15.16 1.73 0.18 1.33 86 23 106 VAL C, 110 LEU C, 300101 THR C 1.22 19.41 2.43 0.5 0.64 86 layer with bottle neck
layer with local minimum
-
show | | profile | lining residues
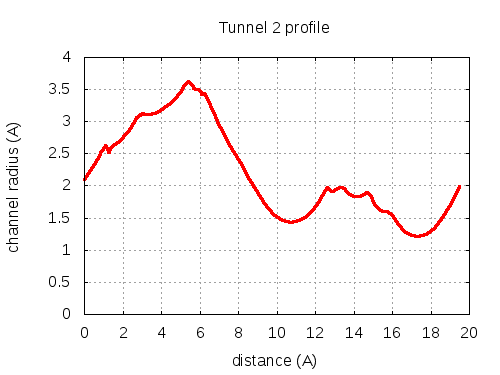
Unique lining residues set - all
73 ALA C, 74 THR C, 75 THR C, 100 ILE C, 103 PHE C, 100 ILE C, 300100 ILE C, 104 GLY C, 200107 THR C, 200103 PHE C, 101 THR C, 200106 VAL C, 200110 LEU C, 101 THR C
Unique lining residues set - sidechains
75 THR C, 103 PHE C, 100 ILE C, 300100 ILE C, 200107 THR C, 200106 VAL C, 200110 LEU C, 101 THR C
Physicochemical properties of lining side-chains
Charge: 0 (0-0)
Hydropathy: 1.6
Hydrophobicity: 0.3
Polarity: 1.49
Mutability: 91
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 73 ALA C, 74 THR C, 75 THR C, 100 ILE C, 103 PHE C 2.11 0.55 0.18 -0.36 2.43 79 2 74 THR C, 75 THR C, 100 ILE C, 103 PHE C 2.34 1.79 0.33 -0.26 2.19 79 3 75 THR C, 103 PHE C, 100 ILE C 2.7 2.21 2.2 0.8 0.71 87 4 75 THR C, 103 PHE C, 100 ILE C, 300100 ILE C 2.84 2.57 2.78 1.05 0.57 91 5 75 THR C, 103 PHE C, 100 ILE C 3 3.02 2.2 0.8 0.71 87 6 75 THR C, 103 PHE C, 100 ILE C, 300100 ILE C 3.12 3.53 2.78 1.05 0.57 91 7 103 PHE C, 100 ILE C, 300100 ILE C 3.13 4.98 3.93 1.66 0.2 85 8 103 PHE C, 100 ILE C, 300100 ILE C, 104 GLY C 3.47 5.61 2.85 1.04 1 85 9 103 PHE C, 100 ILE C, 104 GLY C 3.19 7.04 2.3 0.79 1.29 77 10 100 ILE C, 104 GLY C, 200107 THR C 1.95 10.03 1.13 0.08 1.72 105 11 100 ILE C, 104 GLY C, 200107 THR C, 200103 PHE C 1.5 11.2 0.75 -0.14 2.14 105 12 100 ILE C, 200107 THR C, 200103 PHE C 1.47 12.52 1.13 0.08 1.72 105 13 100 ILE C, 200107 THR C, 200103 PHE C, 101 THR C 1.91 13.15 0.75 -0.14 2.14 105 14 100 ILE C, 200107 THR C, 200103 PHE C, 101 THR C, 200106 VAL C 1.96 13.37 0.46 -0.41 2.39 102 15 200107 THR C, 200103 PHE C, 101 THR C, 200106 VAL C 1.87 14.09 0.68 -0.31 2.14 102 16 200107 THR C, 101 THR C, 200106 VAL C 1.83 14.46 1.03 -0.15 1.72 102 17 200107 THR C, 101 THR C, 200106 VAL C, 200110 LEU C 1.79 15.12 1.73 0.18 1.33 86 18 200106 VAL C, 200110 LEU C, 101 THR C 1.21 19.42 2.43 0.5 0.64 86 layer with bottle neck
layer with local minimum
-
show | | profile | lining residues
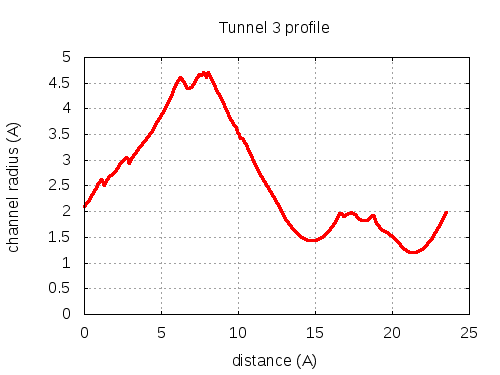
Unique lining residues set - all
73 ALA C, 74 THR C, 75 THR C, 100 ILE C, 103 PHE C, 100 ILE C, 300100 ILE C, 200075 THR C, 300075 THR C, 100075 THR C, 100100 ILE C, 200100 ILE C, 100107 THR C, 300107 THR C, 107 THR C, 200107 THR C, 200104 GLY C, 200103 PHE C, 100103 PHE C, 200101 THR C, 100106 VAL C, 200100 ILE C, 100110 LEU C, 200101 THR C
Unique lining residues set - sidechains
75 THR C, 103 PHE C, 100 ILE C, 300100 ILE C, 200075 THR C, 300075 THR C, 100075 THR C, 100100 ILE C, 200100 ILE C, 100107 THR C, 300107 THR C, 107 THR C, 200107 THR C, 200103 PHE C, 100106 VAL C, 100110 LEU C, 200101 THR C
Physicochemical properties of lining side-chains
Charge: 0 (0-0)
Hydropathy: 1.8
Hydrophobicity: 0.46
Polarity: 1.18
Mutability: 95
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 73 ALA C, 74 THR C, 75 THR C, 100 ILE C, 103 PHE C 2.11 0.61 0.18 -0.36 2.43 79 2 74 THR C, 75 THR C, 100 ILE C, 103 PHE C 2.38 2.05 0.33 -0.26 2.19 79 3 75 THR C, 103 PHE C, 100 ILE C 2.82 2.5 2.2 0.8 0.71 87 4 75 THR C, 103 PHE C, 100 ILE C, 300100 ILE C 2.99 2.81 2.78 1.05 0.57 91 5 103 PHE C, 100 ILE C, 300100 ILE C 2.94 3.19 3.93 1.66 0.2 85 6 75 THR C, 103 PHE C, 100 ILE C, 300100 ILE C 3.12 5.15 2.78 1.05 0.57 91 7 75 THR C, 100 ILE C, 300100 ILE C, 200075 THR C, 300075 THR C 3.99 6.63 1.38 0.26 1.05 105 8 100 ILE C, 300100 ILE C, 100075 THR C, 100100 ILE C, 200100 ILE C 4.39 7.01 3.46 1.29 0.44 103 9 100 ILE C, 300100 ILE C, 100100 ILE C, 200100 ILE C, 100107 THR C 4.48 7.35 3.46 1.29 0.44 103 10 100 ILE C, 100100 ILE C, 200100 ILE C, 100107 THR C, 300107 THR C 4.65 7.53 2.42 0.78 0.74 104 11 100 ILE C, 300100 ILE C, 300107 THR C, 107 THR C, 200107 THR C 4.66 7.66 1.38 0.26 1.05 105 12 100 ILE C, 100100 ILE C, 200100 ILE C, 100107 THR C, 300107 THR C 4.68 7.68 2.42 0.78 0.74 104 13 100 ILE C, 100100 ILE C, 200100 ILE C, 100107 THR C, 200107 THR C 4.66 7.74 2.42 0.78 0.74 104 14 300100 ILE C, 100100 ILE C, 300107 THR C, 107 THR C, 200107 THR C 4.68 7.81 1.38 0.26 1.05 105 15 100 ILE C, 300100 ILE C, 100100 ILE C, 300107 THR C, 107 THR C 4.63 7.82 2.42 0.78 0.74 104 16 300100 ILE C, 100100 ILE C, 100107 THR C, 300107 THR C, 107 THR C 4.63 7.95 1.38 0.26 1.05 105 17 100 ILE C, 200100 ILE C, 100107 THR C, 107 THR C, 200107 THR C 4.69 8.26 1.38 0.26 1.05 105 18 100 ILE C, 200100 ILE C, 100107 THR C, 200107 THR C, 200104 GLY C 4.02 9.34 1.44 0.26 1.39 105 19 100 ILE C, 200100 ILE C, 200107 THR C, 200104 GLY C 3.82 9.56 1.98 0.51 1.33 104 20 100 ILE C, 200100 ILE C, 200104 GLY C, 200103 PHE C 3.57 9.86 2.85 1.04 1 85 21 200100 ILE C, 200104 GLY C, 200103 PHE C 3.17 11.13 2.3 0.79 1.29 77 22 200100 ILE C, 100107 THR C, 200104 GLY C 1.68 14.74 1.13 0.08 1.72 105 23 200100 ILE C, 100107 THR C, 100103 PHE C 1.43 16.45 1.13 0.08 1.72 105 24 200100 ILE C, 100107 THR C, 100103 PHE C, 200101 THR C 1.91 17 0.75 -0.14 2.14 105 25 100107 THR C, 100103 PHE C, 200101 THR C, 100106 VAL C, 200100 ILE C 1.95 17.23 0.46 -0.41 2.39 102 26 100107 THR C, 100103 PHE C, 200101 THR C, 100106 VAL C 1.9 18 0.68 -0.31 2.14 102 27 100107 THR C, 200101 THR C, 100106 VAL C 1.83 18.42 1.03 -0.15 1.72 102 28 100107 THR C, 200101 THR C, 100106 VAL C, 100110 LEU C 1.75 19.23 1.73 0.18 1.33 86 29 100106 VAL C, 100110 LEU C, 200101 THR C 1.23 23.36 2.43 0.5 0.64 86 layer with bottle neck
layer with local minimum
-
show | | profile | lining residues
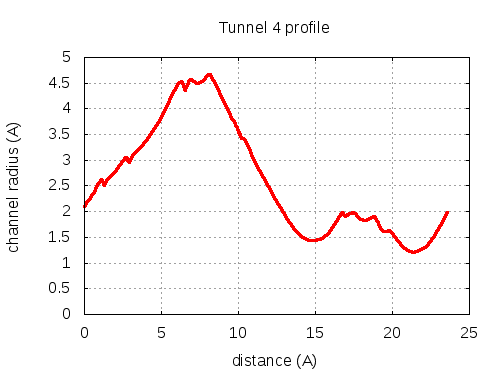
Unique lining residues set - all
73 ALA C, 74 THR C, 75 THR C, 100 ILE C, 103 PHE C, 100 ILE C, 300100 ILE C, 200075 THR C, 300075 THR C, 100100 ILE C, 100075 THR C, 200100 ILE C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100104 GLY C, 100103 PHE C, 300103 PHE C, 100101 THR C, 100100 ILE C, 300106 VAL C, 300110 LEU C, 100101 THR C
Unique lining residues set - sidechains
75 THR C, 103 PHE C, 100 ILE C, 300100 ILE C, 200075 THR C, 300075 THR C, 100100 ILE C, 100075 THR C, 200100 ILE C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100103 PHE C, 300106 VAL C, 300110 LEU C, 100101 THR C
Physicochemical properties of lining side-chains
Charge: 0 (0-0)
Hydropathy: 1.7
Hydrophobicity: 0.4
Polarity: 1.29
Mutability: 95
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 73 ALA C, 74 THR C, 75 THR C, 100 ILE C, 103 PHE C 2.11 0.56 0.18 -0.36 2.43 79 2 74 THR C, 75 THR C, 100 ILE C, 103 PHE C 2.35 2.03 0.33 -0.26 2.19 79 3 75 THR C, 103 PHE C, 100 ILE C 2.8 2.46 2.2 0.8 0.71 87 4 75 THR C, 103 PHE C, 100 ILE C, 300100 ILE C 2.96 2.72 2.78 1.05 0.57 91 5 75 THR C, 103 PHE C, 100 ILE C 2.96 2.95 2.2 0.8 0.71 87 6 75 THR C, 103 PHE C, 100 ILE C, 300100 ILE C 3 5.38 2.78 1.05 0.57 91 7 75 THR C, 100 ILE C, 300100 ILE C, 200075 THR C, 300075 THR C 4.1 6.15 1.38 0.26 1.05 105 8 75 THR C, 100 ILE C, 300100 ILE C, 300075 THR C, 100100 ILE C 4.52 6.47 2.42 0.78 0.74 104 9 200075 THR C, 300075 THR C, 100100 ILE C, 100075 THR C, 200100 ILE C 4.38 6.95 1.38 0.26 1.05 105 10 75 THR C, 100 ILE C, 300100 ILE C, 100100 ILE C, 200100 ILE C 4.5 7.63 3.46 1.29 0.44 103 11 100 ILE C, 300100 ILE C, 100100 ILE C, 200100 ILE C, 107 THR C 4.55 7.98 3.46 1.29 0.44 103 12 100 ILE C, 100100 ILE C, 200100 ILE C, 100107 THR C, 200107 THR C 4.67 8.3 2.42 0.78 0.74 104 13 100100 ILE C, 200100 ILE C, 100107 THR C, 200107 THR C, 300107 THR C 4.55 8.64 1.38 0.26 1.05 105 14 100100 ILE C, 200100 ILE C, 100107 THR C, 300107 THR C, 100104 GLY C 4.13 9.29 1.44 0.26 1.39 105 15 100100 ILE C, 200100 ILE C, 100107 THR C, 100104 GLY C 3.93 9.53 1.98 0.51 1.33 104 16 100100 ILE C, 200100 ILE C, 100104 GLY C, 100103 PHE C 3.57 9.96 2.85 1.04 1 85 17 100100 ILE C, 100104 GLY C, 100103 PHE C 3.14 11.22 2.3 0.79 1.29 77 18 100100 ILE C, 300107 THR C, 100104 GLY C 1.79 14.47 1.13 0.08 1.72 105 19 100100 ILE C, 300107 THR C, 100104 GLY C, 300103 PHE C 1.44 15.6 0.75 -0.14 2.14 105 20 100100 ILE C, 300107 THR C, 300103 PHE C 1.55 16.34 1.13 0.08 1.72 105 21 100100 ILE C, 300107 THR C, 300103 PHE C, 100101 THR C 1.85 17.11 0.75 -0.14 2.14 105 22 300107 THR C, 300103 PHE C, 100101 THR C, 100100 ILE C, 300106 VAL C 1.96 17.33 0.46 -0.41 2.39 102 23 300107 THR C, 300103 PHE C, 100101 THR C, 300106 VAL C 1.91 18.01 0.68 -0.31 2.14 102 24 300107 THR C, 100101 THR C, 300106 VAL C 1.83 18.41 1.03 -0.15 1.72 102 25 300107 THR C, 100101 THR C, 300106 VAL C, 300110 LEU C 1.81 19.12 1.73 0.18 1.33 86 26 300106 VAL C, 300110 LEU C, 100101 THR C 1.21 23.46 2.43 0.5 0.64 86 layer with bottle neck
layer with local minimum
-
show | | profile | lining residues
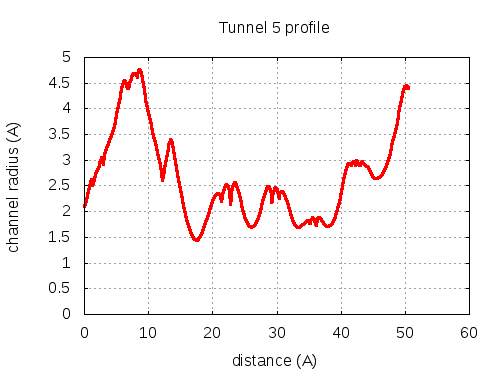
Unique lining residues set - all
73 ALA C, 74 THR C, 75 THR C, 100 ILE C, 103 PHE C, 100 ILE C, 300100 ILE C, 200075 THR C, 300075 THR C, 100075 THR C, 100100 ILE C, 200100 ILE C, 100107 THR C, 107 THR C, 200107 THR C, 300107 THR C, 104 GLY C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C, 124 HIS C, 100124 HIS C, 200124 HIS C, 300124 HIS C
Unique lining residues set - sidechains
75 THR C, 103 PHE C, 100 ILE C, 300100 ILE C, 200075 THR C, 300075 THR C, 100075 THR C, 100100 ILE C, 200100 ILE C, 100107 THR C, 107 THR C, 200107 THR C, 300107 THR C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C, 124 HIS C, 100124 HIS C, 200124 HIS C, 300124 HIS C
Physicochemical properties of lining side-chains
Charge: 0 (0-0)
Hydropathy: 0.8
Hydrophobicity: 0.16
Polarity: 4.81
Mutability: 96
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 73 ALA C, 74 THR C, 75 THR C, 100 ILE C, 103 PHE C 2.11 0.42 0.18 -0.36 2.43 79 2 74 THR C, 75 THR C, 103 PHE C, 100 ILE C 2.28 0.61 1.55 0.4 1.38 87 3 74 THR C, 75 THR C, 100 ILE C, 103 PHE C 2.38 1.86 0.33 -0.26 2.19 79 4 75 THR C, 103 PHE C, 100 ILE C 2.75 2.48 2.2 0.8 0.71 87 5 75 THR C, 103 PHE C, 100 ILE C, 300100 ILE C 2.98 2.68 2.78 1.05 0.57 91 6 75 THR C, 103 PHE C, 100 ILE C 3.05 2.82 2.2 0.8 0.71 87 7 103 PHE C, 100 ILE C, 300100 ILE C 2.93 2.92 3.93 1.66 0.2 85 8 75 THR C, 103 PHE C, 100 ILE C, 300100 ILE C 2.97 5.03 2.78 1.05 0.57 91 9 75 THR C, 100 ILE C, 300100 ILE C, 200075 THR C, 300075 THR C 3.92 6.25 1.38 0.26 1.05 105 10 200075 THR C, 300075 THR C, 100075 THR C, 100100 ILE C, 200100 ILE C 4.55 6.59 1.38 0.26 1.05 105 11 100 ILE C, 300100 ILE C, 100075 THR C, 100100 ILE C, 200100 ILE C 4.42 6.84 3.46 1.29 0.44 103 12 100 ILE C, 300100 ILE C, 100100 ILE C, 200100 ILE C, 100107 THR C 4.4 7.51 3.46 1.29 0.44 103 13 300100 ILE C, 100107 THR C, 107 THR C, 200107 THR C, 300107 THR C 4.67 7.91 0.34 -0.25 1.35 106 14 100 ILE C, 300100 ILE C, 100100 ILE C, 200100 ILE C, 100107 THR C 4.63 8.87 3.46 1.29 0.44 103 15 300100 ILE C, 100107 THR C, 107 THR C, 200107 THR C, 300107 THR C 4.63 9.54 0.34 -0.25 1.35 106 16 100107 THR C, 107 THR C, 200107 THR C, 300107 THR C, 104 GLY C 4.15 10.29 -0.64 -0.78 2 107 17 100107 THR C, 107 THR C, 200107 THR C, 300107 THR C 1.45 19.92 -0.7 -0.77 1.66 107 18 100107 THR C, 107 THR C, 200107 THR C, 300107 THR C, 100111 ALA C 2.22 20.79 -0.2 -0.61 1.33 105 19 107 THR C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.21 22.19 1.3 -0.14 0.33 101 20 100107 THR C, 107 THR C, 200107 THR C, 300107 THR C, 300111 ALA C 2.15 24.11 -0.2 -0.61 1.33 105 21 107 THR C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.26 25.26 1.3 -0.14 0.33 101 22 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 1.75 27.64 1.8 0.02 0 100 23 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300115 VAL C 2.2 28.49 2.28 0.24 0.03 99 24 300111 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 2.17 29.45 3.72 0.91 0.1 98 25 111 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 2.44 29.76 3.72 0.91 0.1 98 26 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300115 VAL C 2.27 30.18 2.28 0.24 0.03 99 27 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 115 VAL C 2.31 30.95 2.28 0.24 0.03 99 28 100111 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 2.33 31.71 3.72 0.91 0.1 98 29 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 1.71 39.38 4.2 1.13 0.13 98 30 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 119 GLN C 2.14 40.99 2.66 0.68 0.81 95 31 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C 2.91 41.83 -3.5 -1.1 3.53 84 32 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 300119 GLN C 2.9 42.13 2.66 0.68 0.81 95 33 200115 VAL C, 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C 3 42.23 -1.96 -0.65 2.85 86 34 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C 2.64 48.67 -3.5 -1.1 3.53 84 35 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C, 124 HIS C 3.86 49.83 -3.44 -0.83 13.14 85 36 200119 GLN C, 124 HIS C, 100124 HIS C, 200124 HIS C, 300124 HIS C 4.43 49.93 -3.26 -0.01 41.99 89 37 300119 GLN C, 124 HIS C, 100124 HIS C, 200124 HIS C, 300124 HIS C 4.41 50.15 -3.26 -0.01 41.99 89 38 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C, 300124 HIS C 4.4 50.24 -3.44 -0.83 13.14 85 39 100119 GLN C, 200119 GLN C, 124 HIS C, 100124 HIS C, 300124 HIS C 4.41 50.24 -3.32 -0.28 32.37 88 layer with bottle neck
layer with local minimum
|
56 pores click to expand / contract
This is an experimental feature.
Merged pores Created by merging tunnels from the selected start point.
No merged pores were found.
Auto pores Computed from pairs of exit points.
-
show | | profile | lining residues
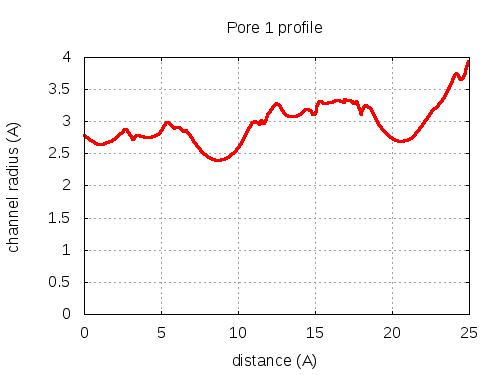
Unique lining residues set - all
300028 SER B, 300030 GLY B, 100082 TYR C, 300058 GLN C, 300092 ASN B, 100084 VAL C, 300064 ARG C, 300032 ASP B, 300031 THR B, 300031 THR B, 100084 VAL C, 300050 TYR B, 300053 GLU B, 100085 THR C, 100085 THR C, 100054 ALA C, 100053 GLY C, 100057 ARG A
Unique lining residues set - sidechains
300028 SER B, 100082 TYR C, 300058 GLN C, 300092 ASN B, 100084 VAL C, 300064 ARG C, 300032 ASP B, 300031 THR B, 300050 TYR B, 300053 GLU B, 100085 THR C, 100054 ALA C, 100057 ARG A
Physicochemical properties of lining side-chains
Charge: 0 (2-2)
Hydropathy: -1.4
Hydrophobicity: -0.41
Polarity: 17.4
Mutability: 88
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 300028 SER B, 300030 GLY B, 100082 TYR C, 300058 GLN C 3.26 0.48 -1.5 -0.44 2.55 83 2 300030 GLY B, 100082 TYR C, 300058 GLN C, 300092 ASN B 3.15 2.57 -2.18 -0.39 2.98 79 3 300030 GLY B, 100082 TYR C, 300092 ASN B 3.26 2.98 -1.73 -0.15 2.79 77 4 300030 GLY B, 100082 TYR C, 300092 ASN B, 100084 VAL C 3.27 3.46 -0.25 0.17 2.13 84 5 300030 GLY B, 100082 TYR C, 300092 ASN B, 100084 VAL C, 300064 ARG C 3.24 3.64 -1.1 0.05 12.1 83 6 300030 GLY B, 300092 ASN B, 100084 VAL C, 300064 ARG C, 300032 ASP B 3.07 5 -1.54 -0.38 21.72 92 7 300030 GLY B, 100084 VAL C, 300064 ARG C, 300032 ASP B 2.99 6.01 -1.05 -0.28 26.3 89 8 300030 GLY B, 100084 VAL C, 300064 ARG C 2.95 6.13 -0.23 -0.03 18.5 90 9 300030 GLY B, 100084 VAL C, 300064 ARG C, 300031 THR B 2.94 6.25 -0.35 -0.22 14.29 96 10 300030 GLY B, 300064 ARG C, 300031 THR B 2.98 6.29 -1.87 -0.66 19.01 95 11 300030 GLY B, 300064 ARG C, 300032 ASP B, 300031 THR B 2.94 6.33 -2.2 -0.77 27.12 84 12 300030 GLY B, 300064 ARG C, 300031 THR B 2.97 6.42 -1.87 -0.66 19.01 95 13 100084 VAL C, 300064 ARG C, 300032 ASP B, 300031 THR B 2.97 6.59 -1.13 -0.28 25.87 93 14 100084 VAL C, 300064 ARG C, 300031 THR B 2.85 6.85 -0.33 -0.02 17.93 96 15 100084 VAL C, 300064 ARG C, 300032 ASP B, 300031 THR B 2.7 7.24 -1.13 -0.28 25.87 93 16 100084 VAL C, 300064 ARG C, 300031 THR B 2.58 7.81 -0.33 -0.02 17.93 96 17 100084 VAL C, 300064 ARG C, 300032 ASP B, 300031 THR B 2.41 10.47 -1.13 -0.28 25.87 93 18 300064 ARG C, 300032 ASP B, 300031 THR B, 100084 VAL C 2.54 11.3 -2.28 -0.76 26.69 92 19 300064 ARG C, 300032 ASP B, 300031 THR B, 100084 VAL C, 300050 TYR B 2.83 11.89 -2.08 -0.38 21.67 81 20 300064 ARG C, 300031 THR B, 100084 VAL C, 300050 TYR B 2.84 12.2 -1.73 -0.22 14.66 80 21 300064 ARG C, 300031 THR B, 300050 TYR B 2.74 12.32 -2.17 -0.03 18.42 80 22 300031 THR B, 300050 TYR B, 300053 GLU B 2.75 12.49 -1.83 -0.27 17.72 78 23 300031 THR B, 100084 VAL C, 300050 TYR B, 300053 GLU B 2.85 13.91 -1.48 -0.4 14.14 78 24 300031 THR B, 100084 VAL C, 300050 TYR B, 300053 GLU B, 100085 THR C 3.07 14.5 -1.26 -0.48 11.99 78 25 300031 THR B, 100084 VAL C, 300053 GLU B, 100085 THR C 3.12 15.23 -1.33 -0.87 14.15 97 26 300031 THR B, 100084 VAL C, 300053 GLU B, 100085 THR C, 100054 ALA C 3.01 16.52 -0.7 -0.69 11.32 97 27 300031 THR B, 300053 GLU B, 100085 THR C, 100054 ALA C 2.9 17.34 -0.78 -0.67 13.31 97 28 300053 GLU B, 100085 THR C, 100054 ALA C 2.86 18.2 -0.8 -0.63 17.19 94 29 300053 GLU B, 100085 THR C, 100054 ALA C, 100053 GLY C 2.98 20.16 -0.7 -0.67 13.74 94 30 300053 GLU B, 100085 THR C, 100054 ALA C, 100053 GLY C, 100057 ARG A 3.59 20.54 -1.46 -0.62 21.39 91 31 300053 GLU B, 100085 THR C, 100053 GLY C, 100057 ARG A 3.8 21.14 -2.28 -0.78 26.74 89 32 300053 GLU B, 100053 GLY C, 100057 ARG A 3.81 21.98 -2.8 -0.79 35.09 80 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
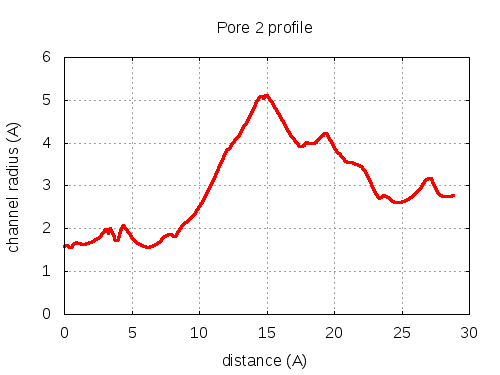
Unique lining residues set - all
200028 SER B, 200030 GLY B, 82 TYR C, 200058 GLN C, 200092 ASN B, 84 VAL C, 200064 ARG C, 200032 ASP B, 200031 THR B, 200031 THR B, 84 VAL C, 200050 TYR B, 200053 GLU B, 85 THR C, 85 THR C, 54 ALA C, 53 GLY C, 57 ARG A
Unique lining residues set - sidechains
200028 SER B, 82 TYR C, 200058 GLN C, 200092 ASN B, 84 VAL C, 200064 ARG C, 200032 ASP B, 200031 THR B, 200050 TYR B, 200053 GLU B, 85 THR C, 54 ALA C, 57 ARG A
Physicochemical properties of lining side-chains
Charge: 0 (2-2)
Hydropathy: -1.4
Hydrophobicity: -0.41
Polarity: 17.4
Mutability: 88
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 200028 SER B, 200030 GLY B, 82 TYR C, 200058 GLN C 3.26 0.48 -1.5 -0.44 2.55 83 2 200030 GLY B, 82 TYR C, 200058 GLN C, 200092 ASN B 3.15 2.57 -2.18 -0.39 2.98 79 3 200030 GLY B, 82 TYR C, 200092 ASN B 3.26 2.98 -1.73 -0.15 2.79 77 4 200030 GLY B, 82 TYR C, 200092 ASN B, 84 VAL C 3.27 3.46 -0.25 0.17 2.13 84 5 200030 GLY B, 82 TYR C, 200092 ASN B, 84 VAL C, 200064 ARG C 3.24 3.64 -1.1 0.05 12.1 83 6 200030 GLY B, 200092 ASN B, 84 VAL C, 200064 ARG C, 200032 ASP B 3.07 5 -1.54 -0.38 21.72 92 7 200030 GLY B, 84 VAL C, 200064 ARG C, 200032 ASP B 2.99 6.01 -1.05 -0.28 26.3 89 8 200030 GLY B, 84 VAL C, 200064 ARG C 2.95 6.13 -0.23 -0.03 18.5 90 9 200030 GLY B, 84 VAL C, 200064 ARG C, 200031 THR B 2.94 6.25 -0.35 -0.22 14.29 96 10 200030 GLY B, 200064 ARG C, 200031 THR B 2.98 6.29 -1.87 -0.66 19.01 95 11 200030 GLY B, 200064 ARG C, 200032 ASP B, 200031 THR B 2.94 6.33 -2.2 -0.77 27.12 84 12 200030 GLY B, 200064 ARG C, 200031 THR B 2.97 6.42 -1.87 -0.66 19.01 95 13 84 VAL C, 200064 ARG C, 200032 ASP B, 200031 THR B 2.97 6.59 -1.13 -0.28 25.87 93 14 84 VAL C, 200064 ARG C, 200031 THR B 2.85 6.85 -0.33 -0.02 17.93 96 15 84 VAL C, 200064 ARG C, 200032 ASP B, 200031 THR B 2.7 7.24 -1.13 -0.28 25.87 93 16 84 VAL C, 200064 ARG C, 200031 THR B 2.58 7.81 -0.33 -0.02 17.93 96 17 84 VAL C, 200064 ARG C, 200032 ASP B, 200031 THR B 2.41 10.47 -1.13 -0.28 25.87 93 18 200064 ARG C, 200032 ASP B, 200031 THR B, 84 VAL C 2.54 11.3 -2.28 -0.76 26.69 92 19 200064 ARG C, 200032 ASP B, 200031 THR B, 84 VAL C, 200050 TYR B 2.83 11.89 -2.08 -0.38 21.67 81 20 200064 ARG C, 200031 THR B, 84 VAL C, 200050 TYR B 2.84 12.2 -1.73 -0.22 14.66 80 21 200064 ARG C, 200031 THR B, 200050 TYR B 2.74 12.32 -2.17 -0.03 18.42 80 22 200031 THR B, 200050 TYR B, 200053 GLU B 2.75 12.49 -1.83 -0.27 17.72 78 23 200031 THR B, 84 VAL C, 200050 TYR B, 200053 GLU B 2.85 13.91 -1.48 -0.4 14.14 78 24 200031 THR B, 84 VAL C, 200050 TYR B, 200053 GLU B, 85 THR C 3.07 14.5 -1.26 -0.48 11.99 78 25 200031 THR B, 84 VAL C, 200053 GLU B, 85 THR C 3.12 15.23 -1.33 -0.87 14.15 97 26 200031 THR B, 84 VAL C, 200053 GLU B, 85 THR C, 54 ALA C 3.01 16.52 -0.7 -0.69 11.32 97 27 200031 THR B, 200053 GLU B, 85 THR C, 54 ALA C 2.9 17.34 -0.78 -0.67 13.31 97 28 200053 GLU B, 85 THR C, 54 ALA C 2.86 18.2 -0.8 -0.63 17.19 94 29 200053 GLU B, 85 THR C, 54 ALA C, 53 GLY C 2.98 20.16 -0.7 -0.67 13.74 94 30 200053 GLU B, 85 THR C, 54 ALA C, 53 GLY C, 57 ARG A 3.59 20.54 -1.46 -0.62 21.39 91 31 200053 GLU B, 85 THR C, 53 GLY C, 57 ARG A 3.8 21.14 -2.28 -0.78 26.74 89 32 200053 GLU B, 53 GLY C, 57 ARG A 3.81 21.98 -2.8 -0.79 35.09 80 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
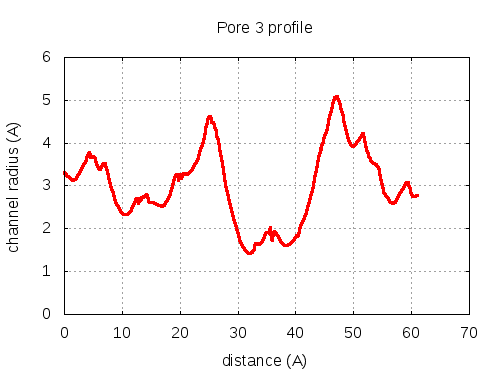
Unique lining residues set - all
100028 SER B, 100030 GLY B, 100058 GLN C, 200082 TYR C, 100092 ASN B, 200084 VAL C, 100064 ARG C, 100032 ASP B, 100031 THR B, 100031 THR B, 200084 VAL C, 100050 TYR B, 100053 GLU B, 200085 THR C, 200085 THR C, 200054 ALA C, 200053 GLY C, 200057 ARG A
Unique lining residues set - sidechains
100028 SER B, 100058 GLN C, 200082 TYR C, 100092 ASN B, 200084 VAL C, 100064 ARG C, 100032 ASP B, 100031 THR B, 100050 TYR B, 100053 GLU B, 200085 THR C, 200054 ALA C, 200057 ARG A
Physicochemical properties of lining side-chains
Charge: 0 (2-2)
Hydropathy: -1.4
Hydrophobicity: -0.41
Polarity: 17.4
Mutability: 88
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 100028 SER B, 100030 GLY B, 100058 GLN C, 200082 TYR C 3.26 0.48 -1.5 -0.44 2.55 83 2 100030 GLY B, 100058 GLN C, 200082 TYR C, 100092 ASN B 3.15 2.57 -2.18 -0.39 2.98 79 3 100030 GLY B, 200082 TYR C, 100092 ASN B 3.26 2.98 -1.73 -0.15 2.79 77 4 100030 GLY B, 200082 TYR C, 100092 ASN B, 200084 VAL C 3.27 3.46 -0.25 0.17 2.13 84 5 100030 GLY B, 200082 TYR C, 100092 ASN B, 200084 VAL C, 100064 ARG C 3.24 3.64 -1.1 0.05 12.1 83 6 100030 GLY B, 100092 ASN B, 200084 VAL C, 100064 ARG C, 100032 ASP B 3.07 5 -1.54 -0.38 21.72 92 7 100030 GLY B, 200084 VAL C, 100064 ARG C, 100032 ASP B 2.99 6.01 -1.05 -0.28 26.3 89 8 100030 GLY B, 200084 VAL C, 100064 ARG C 2.95 6.13 -0.23 -0.03 18.5 90 9 100030 GLY B, 200084 VAL C, 100064 ARG C, 100031 THR B 2.94 6.25 -0.35 -0.22 14.29 96 10 100030 GLY B, 100064 ARG C, 100031 THR B 2.98 6.29 -1.87 -0.66 19.01 95 11 100030 GLY B, 100064 ARG C, 100032 ASP B, 100031 THR B 2.94 6.33 -2.2 -0.77 27.12 84 12 100030 GLY B, 100064 ARG C, 100031 THR B 2.97 6.42 -1.87 -0.66 19.01 95 13 200084 VAL C, 100064 ARG C, 100032 ASP B, 100031 THR B 2.97 6.59 -1.13 -0.28 25.87 93 14 200084 VAL C, 100064 ARG C, 100031 THR B 2.85 6.85 -0.33 -0.02 17.93 96 15 200084 VAL C, 100064 ARG C, 100032 ASP B, 100031 THR B 2.7 7.24 -1.13 -0.28 25.87 93 16 200084 VAL C, 100064 ARG C, 100031 THR B 2.58 7.81 -0.33 -0.02 17.93 96 17 200084 VAL C, 100064 ARG C, 100032 ASP B, 100031 THR B 2.41 10.47 -1.13 -0.28 25.87 93 18 100064 ARG C, 100032 ASP B, 100031 THR B, 200084 VAL C 2.54 11.3 -2.28 -0.76 26.69 92 19 100064 ARG C, 100032 ASP B, 100031 THR B, 200084 VAL C, 100050 TYR B 2.83 11.89 -2.08 -0.38 21.67 81 20 100064 ARG C, 100031 THR B, 200084 VAL C, 100050 TYR B 2.84 12.2 -1.73 -0.22 14.66 80 21 100064 ARG C, 100031 THR B, 100050 TYR B 2.74 12.32 -2.17 -0.03 18.42 80 22 100031 THR B, 100050 TYR B, 100053 GLU B 2.75 12.49 -1.83 -0.27 17.72 78 23 100031 THR B, 200084 VAL C, 100050 TYR B, 100053 GLU B 2.85 13.91 -1.48 -0.4 14.14 78 24 100031 THR B, 200084 VAL C, 100050 TYR B, 100053 GLU B, 200085 THR C 3.07 14.5 -1.26 -0.48 11.99 78 25 100031 THR B, 200084 VAL C, 100053 GLU B, 200085 THR C 3.12 15.23 -1.33 -0.87 14.15 97 26 100031 THR B, 200084 VAL C, 100053 GLU B, 200085 THR C, 200054 ALA C 3.01 16.52 -0.7 -0.69 11.32 97 27 100031 THR B, 100053 GLU B, 200085 THR C, 200054 ALA C 2.9 17.34 -0.78 -0.67 13.31 97 28 100053 GLU B, 200085 THR C, 200054 ALA C 2.86 18.2 -0.8 -0.63 17.19 94 29 100053 GLU B, 200085 THR C, 200054 ALA C, 200053 GLY C 2.98 20.16 -0.7 -0.67 13.74 94 30 100053 GLU B, 200085 THR C, 200054 ALA C, 200053 GLY C, 200057 ARG A 3.59 20.54 -1.46 -0.62 21.39 91 31 100053 GLU B, 200085 THR C, 200053 GLY C, 200057 ARG A 3.8 21.14 -2.28 -0.78 26.74 89 32 100053 GLU B, 200053 GLY C, 200057 ARG A 3.81 21.98 -2.8 -0.79 35.09 80 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
Unique lining residues set - all
100156 THR A, 100041 ASN B, 100155 VAL A, 100154 PRO A, 100113 THR A, 100093 VAL A, 100095 TYR A, 100039 GLN A, 100042 GLY A, 100041 PRO A, 100041 ASN B, 100038 GLN B, 100085 ASN B, 100040 THR B, 100040 THR B, 100165 ASP B, 100103 LYS B, 100010 ILE B
Unique lining residues set - sidechains
100156 THR A, 100041 ASN B, 100154 PRO A, 100113 THR A, 100093 VAL A, 100095 TYR A, 100039 GLN A, 100038 GLN B, 100085 ASN B, 100040 THR B, 100165 ASP B, 100103 LYS B, 100010 ILE B
Physicochemical properties of lining side-chains
Charge: 0 (1-1)
Hydropathy: -1.7
Hydrophobicity: -0.65
Polarity: 7.41
Mutability: 89
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 100156 THR A, 100041 ASN B 2.78 0.83 -2.1 -0.77 2.52 105 2 100156 THR A, 100041 ASN B, 100155 VAL A 2.66 2.73 -1.53 -0.78 2.81 105 3 100156 THR A, 100041 ASN B, 100155 VAL A, 100154 PRO A 2.88 3 -1.55 -0.61 2.5 89 4 100041 ASN B, 100154 PRO A 2.73 5.06 -2.55 -0.43 2.48 81 5 100041 ASN B, 100154 PRO A, 100113 THR A 2.88 5.82 -1.93 -0.54 2.21 89 6 100041 ASN B, 100154 PRO A, 100113 THR A, 100093 VAL A 2.9 6.11 -0.4 -0.13 1.69 91 7 100041 ASN B, 100113 THR A, 100093 VAL A 2.85 6.9 0 -0.14 1.72 103 8 100041 ASN B, 100093 VAL A 2.57 8.37 0.35 0.18 1.76 101 9 100041 ASN B, 100093 VAL A, 100095 TYR A 2.4 10.95 -0.2 0.49 1.71 84 10 100041 ASN B, 100093 VAL A, 100039 GLN A 2.99 11.27 -0.93 -0.25 2.35 95 11 100041 ASN B, 100039 GLN A 2.97 12.23 -3.5 -0.94 3.46 94 12 100041 ASN B, 100039 GLN A, 100042 GLY A 3.24 12.56 -2.47 -0.89 3.43 94 13 100041 ASN B, 100039 GLN A, 100042 GLY A, 100041 PRO A 3.27 13 -1.95 -0.87 3.42 94 14 100041 ASN B, 100039 GLN A, 100042 GLY A 3.11 13.51 -2.47 -0.89 3.43 94 15 100039 GLN A, 100042 GLY A, 100041 ASN B 3.08 14.72 -1.43 -0.9 3.43 84 16 100039 GLN A, 100041 ASN B, 100038 GLN B 3.11 14.97 -2.47 -1 3.48 84 17 100039 GLN A, 100042 GLY A, 100041 ASN B, 100038 GLN B 3.14 15.15 -1.95 -0.95 3.46 84 18 100039 GLN A, 100042 GLY A, 100041 ASN B, 100038 GLN B, 100085 ASN B 3.29 15.83 -2.26 -0.91 3.44 90 19 100042 GLY A, 100041 ASN B, 100085 ASN B, 100040 THR B 3.29 16.55 -1.18 -0.79 3.38 104 20 100042 GLY A, 100041 ASN B, 100085 ASN B, 100040 THR B 3.3 16.86 -1.25 -0.79 2.95 105 21 100042 GLY A, 100085 ASN B, 100040 THR B 3.32 17.42 -1.53 -0.78 2.81 105 22 100042 GLY A, 100085 ASN B, 100040 THR B, 100165 ASP B 3.29 17.53 -2.03 -0.85 14.53 99 23 100042 GLY A, 100085 ASN B, 100165 ASP B 3.28 17.58 -2.47 -0.87 18.82 95 24 100042 GLY A, 100085 ASN B, 100040 THR B, 100165 ASP B 3.29 17.83 -2.03 -0.85 14.53 99 25 100085 ASN B, 100040 THR B, 100165 ASP B 3.11 18.44 -2.57 -0.86 18.25 99 26 100042 GLY A, 100085 ASN B, 100165 ASP B 2.7 23.92 -2.47 -0.87 18.82 95 27 100042 GLY A, 100085 ASN B, 100165 ASP B, 100103 LYS B 3.66 24.72 -2.83 -0.76 26.49 87 28 100042 GLY A, 100165 ASP B, 100103 LYS B, 100010 ILE B 3.81 24.86 -0.83 -0.11 25.68 87 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
Unique lining residues set - all
100165 SER A, 100168 VAL A, 100169 LYS B, 100167 GLY A, 100169 HIS A, 100167 ASP B, 100166 SER A, 100166 SER A, 100170 ASP B, 100169 LYS B, 100140 MET A, 100138 ASN B, 100187 THR A, 100114 THR B, 100138 ASN A, 100139 SER A
Unique lining residues set - sidechains
100169 LYS B, 100169 HIS A, 100167 ASP B, 100166 SER A, 100170 ASP B, 100140 MET A, 100138 ASN B, 100187 THR A, 100114 THR B, 100138 ASN A, 100139 SER A
Physicochemical properties of lining side-chains
Charge: -1 (1-2)
Hydropathy: -1.4
Hydrophobicity: -0.5
Polarity: 17.57
Mutability: 98
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 100165 SER A, 100168 VAL A, 100169 LYS B 1.57 0.75 -1.57 -0.67 18.75 72 2 100165 SER A, 100168 VAL A, 100169 LYS B, 100167 GLY A 1.65 2.05 -1.28 -0.7 14.91 72 3 100168 VAL A, 100169 LYS B, 100167 GLY A 1.69 2.94 -1.57 -0.67 18.75 72 4 100168 VAL A, 100169 LYS B, 100167 GLY A, 100169 HIS A 1.94 3.18 -1.98 -0.44 26.97 81 5 100168 VAL A, 100169 LYS B, 100167 GLY A, 100169 HIS A 1.9 3.36 -1.98 -0.44 26.97 81 6 100168 VAL A, 100169 LYS B, 100167 GLY A, 100169 HIS A 1.99 3.47 -1.98 -0.44 26.97 81 7 100168 VAL A, 100169 LYS B, 100167 GLY A, 100169 HIS A 1.81 3.8 -1.98 -0.44 26.97 81 8 100168 VAL A, 100167 GLY A, 100169 HIS A, 100167 ASP B 1.73 3.97 -1.88 -0.6 27.02 88 9 100169 LYS B, 100167 GLY A, 100169 HIS A, 100167 ASP B 1.75 4.82 -2.75 -0.5 38.55 83 10 100169 LYS B, 100167 GLY A, 100167 ASP B 1.63 6.39 -2.6 -0.75 34.19 79 11 100169 LYS B, 100167 GLY A, 100166 SER A 1.58 8.05 -1.57 -0.67 18.75 72 12 100169 LYS B, 100167 GLY A, 100166 SER A 1.81 8.24 -1.7 -0.73 18.18 94 13 100169 LYS B, 100166 SER A 1.85 8.49 -2.35 -0.69 25.59 94 14 100169 LYS B, 100167 GLY A, 100166 SER A 1.96 11.59 -1.7 -0.73 18.18 94 15 100169 LYS B, 100167 GLY A, 100166 SER A, 100170 ASP B 3.53 13.1 -2.15 -0.81 26.06 91 16 100167 GLY A, 100166 SER A, 100170 ASP B, 100169 LYS B 4.28 13.62 -1.28 -0.9 14.53 101 17 100167 GLY A, 100166 SER A, 100170 ASP B 4.57 14.49 -1.57 -0.94 18.25 101 18 100167 GLY A, 100166 SER A, 100170 ASP B, 100140 MET A 5.02 15.37 -0.7 -0.45 14.05 98 19 100167 GLY A, 100170 ASP B, 100140 MET A 4.2 17.11 -0.67 -0.28 18.17 89 20 100167 GLY A, 100170 ASP B, 100140 MET A, 100138 ASN B 3.91 18.47 -1.38 -0.4 14.47 94 21 100170 ASP B, 100140 MET A, 100138 ASN B 4 19.67 -1.7 -0.27 18.17 94 22 100140 MET A, 100138 ASN B, 100187 THR A 3.52 21.81 -0.77 -0.18 2.16 101 23 100140 MET A, 100138 ASN B, 100187 THR A, 100114 THR B 2.76 23.47 -0.75 -0.33 2.03 102 24 100140 MET A, 100187 THR A, 100114 THR B 2.61 25.52 0.17 -0.18 1.58 102 25 100140 MET A, 100114 THR B 2.71 26.28 0.6 0.12 1.55 100 26 100140 MET A, 100114 THR B, 100138 ASN A 2.94 26.88 -0.77 -0.18 2.16 101 27 100140 MET A, 100114 THR B, 100138 ASN A, 100139 SER A 2.9 27.78 -0.78 -0.38 2.04 105 28 100140 MET A, 100114 THR B, 100138 ASN A 2.74 28.71 -0.77 -0.18 2.16 101 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
Unique lining residues set - all
100046 GLU A, 100061 ASN A, 100063 LYS A, 100003 LEU B, 100098 PHE B, 100064 ILE A, 300020 SER B, 100063 LYS A, 300018 ARG B, 100040 ARG A, 100088 SER A, 100091 SER A, 100091 SER A, 100041 PRO A, 100088 SER A, 100175 LEU A, 100180 TYR A, 100173 ALA A, 100153 GLU A, 100172 PRO A, 100041 ASN B, 100172 PRO A, 100171 PHE A, 100182 LEU A, 100170 THR A, 100155 VAL A, 100156 THR A, 100157 VAL A, 100040 THR B
Unique lining residues set - sidechains
100046 GLU A, 100061 ASN A, 100063 LYS A, 100003 LEU B, 100064 ILE A, 300020 SER B, 300018 ARG B, 100040 ARG A, 100091 SER A, 100041 PRO A, 100088 SER A, 100175 LEU A, 100180 TYR A, 100153 GLU A, 100172 PRO A, 100041 ASN B, 100182 LEU A, 100170 THR A, 100156 THR A,
Physicochemical properties of lining side-chains
Charge: 1 (3-2)
Hydropathy: -1.2
Hydrophobicity: -0.36
Polarity: 12.09
Mutability: 84
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 100046 GLU A, 100061 ASN A, 100063 LYS A, 100003 LEU B, 100098 PHE B 4.26 1.13 -1.5 -0.4 21.26 76 2 100046 GLU A, 100063 LYS A, 100003 LEU B, 100098 PHE B 4.28 2.22 -1 -0.3 25.73 67 3 100046 GLU A, 100063 LYS A, 100003 LEU B, 100064 ILE A 4.39 5.15 0.23 0.35 24.92 76 4 100046 GLU A, 100063 LYS A, 100003 LEU B, 300020 SER B 5.72 6.18 -1.1 -0.35 25.3 80 5 100046 GLU A, 100063 LYS A, 100064 ILE A 2.91 11.16 -0.97 0.09 33.18 84 6 100046 GLU A, 100064 ILE A, 100063 LYS A 3.3 11.82 0.2 -0.04 17.8 90 7 100046 GLU A, 100064 ILE A, 100063 LYS A, 300018 ARG B 3.62 14.5 -0.98 -0.14 26.35 87 8 100046 GLU A, 100064 ILE A, 300018 ARG B 3.63 14.98 -1.17 0.08 34.01 87 9 100046 GLU A, 100064 ILE A, 300018 ARG B, 100040 ARG A 3.53 18.3 -2 -0.04 38.51 86 10 100046 GLU A, 300018 ARG B, 100040 ARG A 4 20.56 -4.17 -0.66 51.3 81 11 100046 GLU A, 100064 ILE A, 100040 ARG A 4.06 21.34 -1.17 0.08 34.01 87 12 100046 GLU A, 100040 ARG A 2.89 24.13 -4 -0.78 50.95 80 13 100040 ARG A -0.27 30.72 -4.5 -0.42 52 83 14 100040 ARG A, 100088 SER A 1.51 31.47 -2.45 -0.61 27.69 83 15 100040 ARG A, 100088 SER A, 100091 SER A 2.16 32.2 -1.77 -0.67 19.59 83 16 100040 ARG A, 100088 SER A, 100091 SER A 2.32 32.39 -1.9 -0.73 19.02 100 17 100040 ARG A, 100088 SER A, 100091 SER A, 100041 PRO A 2.48 32.62 -1.83 -0.57 14.66 86 18 100040 ARG A, 100088 SER A, 100091 SER A, 100041 PRO A 2.71 33.23 -1.73 -0.53 15.09 70 19 100040 ARG A, 100088 SER A, 100041 PRO A 3.01 35.82 -2.17 -0.44 18.99 70 20 100040 ARG A, 100041 PRO A, 100088 SER A 4.49 37.87 -2.3 -0.49 18.42 86 21 100041 PRO A, 100088 SER A 4.81 39.01 -1.2 -0.53 1.63 87 22 100091 SER A, 100041 PRO A, 100088 SER A 4.57 39.81 -1.07 -0.68 1.64 97 23 100091 SER A, 100041 PRO A, 100088 SER A, 100175 LEU A 4.53 40.44 0.15 -0.22 1.26 86 24 100041 PRO A, 100088 SER A, 100175 LEU A, 100180 TYR A 4.56 40.65 0.02 0.3 1.25 69 25 100091 SER A, 100088 SER A, 100175 LEU A, 100180 TYR A 4.34 40.98 0.23 0.08 1.27 84 26 100091 SER A, 100088 SER A, 100180 TYR A 4.07 41.15 -0.97 -0.28 1.65 94 27 100091 SER A, 100088 SER A 4.13 41.29 -0.8 -0.97 1.67 117 28 100091 SER A, 100088 SER A, 100180 TYR A 4.3 41.6 -0.97 -0.28 1.65 94 29 100091 SER A, 100088 SER A, 100175 LEU A, 100180 TYR A 4.4 42.1 0.23 0.08 1.27 84 30 100088 SER A, 100091 SER A, 100041 PRO A, 100175 LEU A, 100180 TYR A 4.35 42.78 -0.06 0.08 1.67 69 31 100041 PRO A, 100175 LEU A, 100180 TYR A 4.04 47.08 0.3 0.72 1.11 54 32 100041 PRO A, 100175 LEU A, 100180 TYR A, 100173 ALA A 4.46 47.41 0.13 0.34 1.68 54 33 100041 PRO A, 100175 LEU A, 100180 TYR A 4.37 48.04 0.3 0.72 1.11 54 34 100041 PRO A, 100175 LEU A, 100180 TYR A, 100173 ALA A 4.44 48.64 0.13 0.34 1.68 54 35 100041 PRO A, 100180 TYR A, 100173 ALA A 3.47 50.97 -1.1 0.07 2.19 54 36 100041 PRO A, 100180 TYR A, 100173 ALA A, 100153 GLU A 3.28 52.87 -1.7 -0.23 14.12 61 37 100041 PRO A, 100173 ALA A, 100153 GLU A, 100172 PRO A 3.35 55.01 -1.78 -0.53 14.11 64 38 100041 PRO A, 100153 GLU A, 100172 PRO A, 100041 ASN B 3.38 55.73 -2.55 -0.52 14.11 74 39 100041 PRO A, 100153 GLU A, 100172 PRO A, 100041 ASN B 3.47 56.22 -2.55 -0.52 14.11 74 40 100153 GLU A, 100172 PRO A, 100041 ASN B 3.11 56.87 -2.87 -0.67 18.29 79 41 100173 ALA A, 100153 GLU A, 100172 PRO A, 100041 ASN B 2.27 60.27 -2.25 -0.7 14.56 79 42 100173 ALA A, 100153 GLU A, 100041 ASN B, 100172 PRO A 2.44 60.7 -1.95 -0.88 15.01 90 43 100173 ALA A, 100153 GLU A, 100041 ASN B, 100172 PRO A, 100171 PHE A 2.62 61.04 -1.64 -0.86 12.68 90 44 100173 ALA A, 100153 GLU A, 100041 ASN B, 100172 PRO A, 100182 LEU A 2.74 61.28 -0.8 -0.47 12.03 78 45 100153 GLU A, 100041 ASN B, 100172 PRO A, 100171 PHE A, 100182 LEU A 2.62 61.48 -0.8 -0.47 12.03 78 46 100041 ASN B, 100172 PRO A, 100171 PHE A, 100182 LEU A 2.58 62.31 -0.13 -0.31 2.57 79 47 100041 ASN B, 100171 PHE A, 100182 LEU A 2.75 62.63 -0.03 -0.14 2.3 79 48 100041 ASN B, 100182 LEU A, 100170 THR A 2.76 62.87 -0.13 -0.13 1.72 88 49 100041 ASN B, 100182 LEU A, 100170 THR A, 100155 VAL A 2.61 64.04 -0.2 -0.3 2.14 88 50 100041 ASN B, 100170 THR A, 100155 VAL A 2.5 68.13 -1.53 -0.78 2.81 105 51 100041 ASN B, 100156 THR A, 100157 VAL A 3.14 68.5 -1.53 -0.78 2.81 105 52 100041 ASN B, 100170 THR A, 100156 THR A, 100157 VAL A 3.22 68.76 -1.33 -0.78 2.52 106 53 100041 ASN B, 100170 THR A, 100157 VAL A 3.23 68.84 -1.53 -0.78 2.81 105 54 100041 ASN B, 100170 THR A, 100155 VAL A 3.17 69.01 -1.53 -0.78 2.81 105 55 100041 ASN B, 100170 THR A, 100157 VAL A 3.23 69.22 -1.53 -0.78 2.81 105 56 100041 ASN B, 100170 THR A, 100156 THR A, 100157 VAL A 3.19 69.64 -1.33 -0.78 2.52 106 57 100170 THR A, 100156 THR A, 100157 VAL A 3.23 69.79 -0.6 -0.78 2.23 107 58 100041 ASN B, 100170 THR A, 100156 THR A, 100157 VAL A 3.29 70.37 -1.33 -0.78 2.52 106 59 100041 ASN B, 100156 THR A, 100157 VAL A 3.31 72.4 -1.53 -0.78 2.81 105 60 100041 ASN B, 100156 THR A, 100157 VAL A, 100040 THR B 3.79 72.99 -1.25 -0.79 2.95 105 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
Unique lining residues set - all
200046 GLU A, 200061 ASN A, 200063 LYS A, 200003 LEU B, 200098 PHE B, 200064 ILE A, 100020 SER B, 200063 LYS A, 100018 ARG B, 200040 ARG A, 200088 SER A, 200091 SER A, 200091 SER A, 200041 PRO A, 200088 SER A, 200175 LEU A, 200180 TYR A, 200173 ALA A, 200153 GLU A, 200172 PRO A, 200041 ASN B, 200172 PRO A, 200171 PHE A, 200182 LEU A, 200170 THR A, 200155 VAL A, 200156 THR A, 200157 VAL A, 200040 THR B
Unique lining residues set - sidechains
200046 GLU A, 200061 ASN A, 200063 LYS A, 200003 LEU B, 200064 ILE A, 100020 SER B, 100018 ARG B, 200040 ARG A, 200091 SER A, 200041 PRO A, 200088 SER A, 200175 LEU A, 200180 TYR A, 200153 GLU A, 200172 PRO A, 200041 ASN B, 200182 LEU A, 200170 THR A, 200156 THR A,
Physicochemical properties of lining side-chains
Charge: 1 (3-2)
Hydropathy: -1.2
Hydrophobicity: -0.36
Polarity: 12.09
Mutability: 84
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 200046 GLU A, 200061 ASN A, 200063 LYS A, 200003 LEU B, 200098 PHE B 4.26 1.13 -1.5 -0.4 21.26 76 2 200046 GLU A, 200063 LYS A, 200003 LEU B, 200098 PHE B 4.28 2.22 -1 -0.3 25.73 67 3 200046 GLU A, 200063 LYS A, 200003 LEU B, 200064 ILE A 4.39 5.15 0.23 0.35 24.92 76 4 200046 GLU A, 200063 LYS A, 200003 LEU B, 100020 SER B 5.72 6.18 -1.1 -0.35 25.3 80 5 200046 GLU A, 200063 LYS A, 200064 ILE A 2.91 11.16 -0.97 0.09 33.18 84 6 200046 GLU A, 200064 ILE A, 200063 LYS A 3.3 11.82 0.2 -0.04 17.8 90 7 200046 GLU A, 200064 ILE A, 200063 LYS A, 100018 ARG B 3.62 14.5 -0.98 -0.14 26.35 87 8 200046 GLU A, 200064 ILE A, 100018 ARG B 3.63 14.98 -1.17 0.08 34.01 87 9 200046 GLU A, 200064 ILE A, 100018 ARG B, 200040 ARG A 3.53 18.3 -2 -0.04 38.51 86 10 200046 GLU A, 100018 ARG B, 200040 ARG A 4 20.56 -4.17 -0.66 51.3 81 11 200046 GLU A, 200064 ILE A, 200040 ARG A 4.06 21.34 -1.17 0.08 34.01 87 12 200046 GLU A, 200040 ARG A 2.89 24.13 -4 -0.78 50.95 80 13 200040 ARG A -0.27 30.72 -4.5 -0.42 52 83 14 200040 ARG A, 200088 SER A 1.51 31.47 -2.45 -0.61 27.69 83 15 200040 ARG A, 200088 SER A, 200091 SER A 2.16 32.2 -1.77 -0.67 19.59 83 16 200040 ARG A, 200088 SER A, 200091 SER A 2.32 32.39 -1.9 -0.73 19.02 100 17 200040 ARG A, 200088 SER A, 200091 SER A, 200041 PRO A 2.48 32.62 -1.83 -0.57 14.66 86 18 200040 ARG A, 200088 SER A, 200091 SER A, 200041 PRO A 2.71 33.23 -1.73 -0.53 15.09 70 19 200040 ARG A, 200088 SER A, 200041 PRO A 3.01 35.82 -2.17 -0.44 18.99 70 20 200040 ARG A, 200041 PRO A, 200088 SER A 4.49 37.87 -2.3 -0.49 18.42 86 21 200041 PRO A, 200088 SER A 4.81 39.01 -1.2 -0.53 1.63 87 22 200091 SER A, 200041 PRO A, 200088 SER A 4.57 39.81 -1.07 -0.68 1.64 97 23 200091 SER A, 200041 PRO A, 200088 SER A, 200175 LEU A 4.53 40.44 0.15 -0.22 1.26 86 24 200041 PRO A, 200088 SER A, 200175 LEU A, 200180 TYR A 4.56 40.65 0.02 0.3 1.25 69 25 200091 SER A, 200088 SER A, 200175 LEU A, 200180 TYR A 4.34 40.98 0.23 0.08 1.27 84 26 200091 SER A, 200088 SER A, 200180 TYR A 4.07 41.15 -0.97 -0.28 1.65 94 27 200091 SER A, 200088 SER A 4.13 41.29 -0.8 -0.97 1.67 117 28 200091 SER A, 200088 SER A, 200180 TYR A 4.3 41.6 -0.97 -0.28 1.65 94 29 200091 SER A, 200088 SER A, 200175 LEU A, 200180 TYR A 4.4 42.11 0.23 0.08 1.27 84 30 200088 SER A, 200091 SER A, 200041 PRO A, 200175 LEU A, 200180 TYR A 4.35 42.78 -0.06 0.08 1.67 69 31 200041 PRO A, 200175 LEU A, 200180 TYR A 4.04 47.08 0.3 0.72 1.11 54 32 200041 PRO A, 200175 LEU A, 200180 TYR A, 200173 ALA A 4.46 47.42 0.13 0.34 1.68 54 33 200041 PRO A, 200175 LEU A, 200180 TYR A 4.37 48.05 0.3 0.72 1.11 54 34 200041 PRO A, 200175 LEU A, 200180 TYR A, 200173 ALA A 4.44 48.64 0.13 0.34 1.68 54 35 200041 PRO A, 200180 TYR A, 200173 ALA A 3.47 50.97 -1.1 0.07 2.19 54 36 200041 PRO A, 200180 TYR A, 200173 ALA A, 200153 GLU A 3.28 52.87 -1.7 -0.23 14.12 61 37 200041 PRO A, 200173 ALA A, 200153 GLU A, 200172 PRO A 3.35 55.01 -1.78 -0.53 14.11 64 38 200041 PRO A, 200153 GLU A, 200172 PRO A, 200041 ASN B 3.38 55.73 -2.55 -0.52 14.11 74 39 200041 PRO A, 200153 GLU A, 200172 PRO A, 200041 ASN B 3.47 56.22 -2.55 -0.52 14.11 74 40 200153 GLU A, 200172 PRO A, 200041 ASN B 3.11 56.87 -2.87 -0.67 18.29 79 41 200173 ALA A, 200153 GLU A, 200172 PRO A, 200041 ASN B 2.27 60.27 -2.25 -0.7 14.56 79 42 200173 ALA A, 200153 GLU A, 200041 ASN B, 200172 PRO A 2.44 60.7 -1.95 -0.88 15.01 90 43 200173 ALA A, 200153 GLU A, 200041 ASN B, 200172 PRO A, 200171 PHE A 2.62 61.04 -1.64 -0.86 12.68 90 44 200173 ALA A, 200153 GLU A, 200041 ASN B, 200172 PRO A, 200182 LEU A 2.74 61.28 -0.8 -0.47 12.03 78 45 200153 GLU A, 200041 ASN B, 200172 PRO A, 200171 PHE A, 200182 LEU A 2.62 61.48 -0.8 -0.47 12.03 78 46 200041 ASN B, 200172 PRO A, 200171 PHE A, 200182 LEU A 2.58 62.31 -0.13 -0.31 2.57 79 47 200041 ASN B, 200171 PHE A, 200182 LEU A 2.75 62.63 -0.03 -0.14 2.3 79 48 200041 ASN B, 200182 LEU A, 200170 THR A 2.76 62.87 -0.13 -0.13 1.72 88 49 200041 ASN B, 200182 LEU A, 200170 THR A, 200155 VAL A 2.61 64.04 -0.2 -0.3 2.14 88 50 200041 ASN B, 200170 THR A, 200155 VAL A 2.5 68.13 -1.53 -0.78 2.81 105 51 200041 ASN B, 200156 THR A, 200157 VAL A 3.14 68.5 -1.53 -0.78 2.81 105 52 200041 ASN B, 200170 THR A, 200156 THR A, 200157 VAL A 3.22 68.76 -1.33 -0.78 2.52 106 53 200041 ASN B, 200170 THR A, 200157 VAL A 3.23 68.84 -1.53 -0.78 2.81 105 54 200041 ASN B, 200170 THR A, 200155 VAL A 3.17 69.01 -1.53 -0.78 2.81 105 55 200041 ASN B, 200170 THR A, 200157 VAL A 3.23 69.23 -1.53 -0.78 2.81 105 56 200041 ASN B, 200170 THR A, 200156 THR A, 200157 VAL A 3.19 69.65 -1.33 -0.78 2.52 106 57 200170 THR A, 200156 THR A, 200157 VAL A 3.23 69.79 -0.6 -0.78 2.23 107 58 200041 ASN B, 200170 THR A, 200156 THR A, 200157 VAL A 3.29 70.38 -1.33 -0.78 2.52 106 59 200041 ASN B, 200156 THR A, 200157 VAL A 3.31 72.4 -1.53 -0.78 2.81 105 60 200041 ASN B, 200156 THR A, 200157 VAL A, 200040 THR B 3.79 72.99 -1.25 -0.79 2.95 105 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
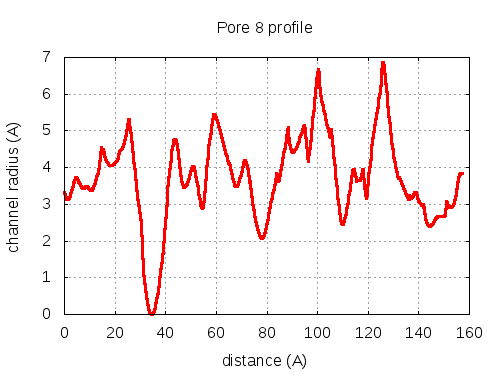
Unique lining residues set - all
46 GLU A, 61 ASN A, 63 LYS A, 3 LEU B, 98 PHE B, 64 ILE A, 200020 SER B, 63 LYS A, 200018 ARG B, 40 ARG A, 88 SER A, 91 SER A, 91 SER A, 41 PRO A, 88 SER A, 175 LEU A, 180 TYR A, 173 ALA A, 153 GLU A, 172 PRO A, 41 ASN B, 172 PRO A, 171 PHE A, 182 LEU A, 170 THR A, 155 VAL A, 156 THR A, 157 VAL A, 40 THR B
Unique lining residues set - sidechains
46 GLU A, 61 ASN A, 63 LYS A, 3 LEU B, 64 ILE A, 200020 SER B, 200018 ARG B, 40 ARG A, 91 SER A, 41 PRO A, 88 SER A, 175 LEU A, 180 TYR A, 153 GLU A, 172 PRO A, 41 ASN B, 182 LEU A, 170 THR A, 156 THR A,
Physicochemical properties of lining side-chains
Charge: 1 (3-2)
Hydropathy: -1.2
Hydrophobicity: -0.36
Polarity: 12.09
Mutability: 84
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 46 GLU A, 61 ASN A, 63 LYS A, 3 LEU B, 98 PHE B 4.26 1.13 -1.5 -0.4 21.26 76 2 46 GLU A, 63 LYS A, 3 LEU B, 98 PHE B 4.28 2.22 -1 -0.3 25.73 67 3 46 GLU A, 63 LYS A, 3 LEU B, 64 ILE A 4.39 5.15 0.23 0.35 24.92 76 4 46 GLU A, 63 LYS A, 3 LEU B, 200020 SER B 5.72 6.18 -1.1 -0.35 25.3 80 5 46 GLU A, 63 LYS A, 64 ILE A 2.91 11.16 -0.97 0.09 33.18 84 6 46 GLU A, 64 ILE A, 63 LYS A 3.3 11.82 0.2 -0.04 17.8 90 7 46 GLU A, 64 ILE A, 63 LYS A, 200018 ARG B 3.62 14.5 -0.98 -0.14 26.35 87 8 46 GLU A, 64 ILE A, 200018 ARG B 3.63 14.98 -1.17 0.08 34.01 87 9 46 GLU A, 64 ILE A, 200018 ARG B, 40 ARG A 3.53 18.3 -2 -0.04 38.51 86 10 46 GLU A, 200018 ARG B, 40 ARG A 4 20.56 -4.17 -0.66 51.3 81 11 46 GLU A, 64 ILE A, 40 ARG A 4.06 21.34 -1.17 0.08 34.01 87 12 46 GLU A, 40 ARG A 2.89 24.13 -4 -0.78 50.95 80 13 40 ARG A -0.27 30.72 -4.5 -0.42 52 83 14 40 ARG A, 88 SER A 1.51 31.47 -2.45 -0.61 27.69 83 15 40 ARG A, 88 SER A, 91 SER A 2.16 32.2 -1.77 -0.67 19.59 83 16 40 ARG A, 88 SER A, 91 SER A 2.32 32.39 -1.9 -0.73 19.02 100 17 40 ARG A, 88 SER A, 91 SER A, 41 PRO A 2.48 32.62 -1.83 -0.57 14.66 86 18 40 ARG A, 88 SER A, 91 SER A, 41 PRO A 2.71 33.23 -1.73 -0.53 15.09 70 19 40 ARG A, 88 SER A, 41 PRO A 3.01 35.82 -2.17 -0.44 18.99 70 20 40 ARG A, 41 PRO A, 88 SER A 4.49 37.88 -2.3 -0.49 18.42 86 21 41 PRO A, 88 SER A 4.81 39.01 -1.2 -0.53 1.63 87 22 91 SER A, 41 PRO A, 88 SER A 4.57 39.81 -1.07 -0.68 1.64 97 23 91 SER A, 41 PRO A, 88 SER A, 175 LEU A 4.53 40.44 0.15 -0.22 1.26 86 24 41 PRO A, 88 SER A, 175 LEU A, 180 TYR A 4.56 40.66 0.02 0.3 1.25 69 25 91 SER A, 88 SER A, 175 LEU A, 180 TYR A 4.34 40.99 0.23 0.08 1.27 84 26 91 SER A, 88 SER A, 180 TYR A 4.07 41.15 -0.97 -0.28 1.65 94 27 91 SER A, 88 SER A 4.13 41.29 -0.8 -0.97 1.67 117 28 91 SER A, 88 SER A, 180 TYR A 4.3 41.6 -0.97 -0.28 1.65 94 29 91 SER A, 88 SER A, 175 LEU A, 180 TYR A 4.4 42.11 0.23 0.08 1.27 84 30 88 SER A, 91 SER A, 41 PRO A, 175 LEU A, 180 TYR A 4.35 42.78 -0.06 0.08 1.67 69 31 41 PRO A, 175 LEU A, 180 TYR A 4.04 47.08 0.3 0.72 1.11 54 32 41 PRO A, 175 LEU A, 180 TYR A, 173 ALA A 4.46 47.42 0.13 0.34 1.68 54 33 41 PRO A, 175 LEU A, 180 TYR A 4.37 48.05 0.3 0.72 1.11 54 34 41 PRO A, 175 LEU A, 180 TYR A, 173 ALA A 4.44 48.64 0.13 0.34 1.68 54 35 41 PRO A, 180 TYR A, 173 ALA A 3.47 50.97 -1.1 0.07 2.19 54 36 41 PRO A, 180 TYR A, 173 ALA A, 153 GLU A 3.28 52.87 -1.7 -0.23 14.12 61 37 41 PRO A, 173 ALA A, 153 GLU A, 172 PRO A 3.35 55.01 -1.78 -0.53 14.11 64 38 41 PRO A, 153 GLU A, 172 PRO A, 41 ASN B 3.38 55.73 -2.55 -0.52 14.11 74 39 41 PRO A, 153 GLU A, 172 PRO A, 41 ASN B 3.47 56.22 -2.55 -0.52 14.11 74 40 153 GLU A, 172 PRO A, 41 ASN B 3.11 56.88 -2.87 -0.67 18.29 79 41 173 ALA A, 153 GLU A, 172 PRO A, 41 ASN B 2.27 60.27 -2.25 -0.7 14.56 79 42 173 ALA A, 153 GLU A, 41 ASN B, 172 PRO A 2.44 60.7 -1.95 -0.88 15.01 90 43 173 ALA A, 153 GLU A, 41 ASN B, 172 PRO A, 171 PHE A 2.62 61.04 -1.64 -0.86 12.68 90 44 173 ALA A, 153 GLU A, 41 ASN B, 172 PRO A, 182 LEU A 2.74 61.28 -0.8 -0.47 12.03 78 45 153 GLU A, 41 ASN B, 172 PRO A, 171 PHE A, 182 LEU A 2.62 61.48 -0.8 -0.47 12.03 78 46 41 ASN B, 172 PRO A, 171 PHE A, 182 LEU A 2.58 62.31 -0.13 -0.31 2.57 79 47 41 ASN B, 171 PHE A, 182 LEU A 2.75 62.63 -0.03 -0.14 2.3 79 48 41 ASN B, 182 LEU A, 170 THR A 2.76 62.87 -0.13 -0.13 1.72 88 49 41 ASN B, 182 LEU A, 170 THR A, 155 VAL A 2.61 64.04 -0.2 -0.3 2.14 88 50 41 ASN B, 170 THR A, 155 VAL A 2.5 68.13 -1.53 -0.78 2.81 105 51 41 ASN B, 156 THR A, 157 VAL A 3.14 68.5 -1.53 -0.78 2.81 105 52 41 ASN B, 170 THR A, 156 THR A, 157 VAL A 3.22 68.76 -1.33 -0.78 2.52 106 53 41 ASN B, 170 THR A, 157 VAL A 3.23 68.84 -1.53 -0.78 2.81 105 54 41 ASN B, 170 THR A, 155 VAL A 3.17 69.01 -1.53 -0.78 2.81 105 55 41 ASN B, 170 THR A, 157 VAL A 3.23 69.23 -1.53 -0.78 2.81 105 56 41 ASN B, 170 THR A, 156 THR A, 157 VAL A 3.19 69.65 -1.33 -0.78 2.52 106 57 170 THR A, 156 THR A, 157 VAL A 3.23 69.79 -0.6 -0.78 2.23 107 58 41 ASN B, 170 THR A, 156 THR A, 157 VAL A 3.29 70.38 -1.33 -0.78 2.52 106 59 41 ASN B, 156 THR A, 157 VAL A 3.31 72.4 -1.53 -0.78 2.81 105 60 41 ASN B, 156 THR A, 157 VAL A, 40 THR B 3.79 72.99 -1.25 -0.79 2.95 105 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
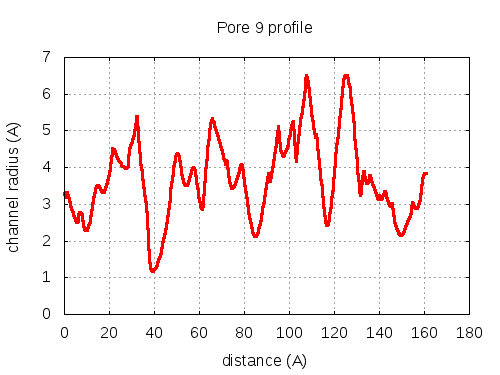
Unique lining residues set - all
300046 GLU A, 300061 ASN A, 300063 LYS A, 300003 LEU B, 300098 PHE B, 300064 ILE A, 20 SER B, 300063 LYS A, 18 ARG B, 300040 ARG A, 300088 SER A, 300091 SER A, 300091 SER A, 300041 PRO A, 300088 SER A, 300175 LEU A, 300180 TYR A, 300173 ALA A, 300153 GLU A, 300172 PRO A, 300041 ASN B, 300172 PRO A, 300171 PHE A, 300182 LEU A, 300170 THR A, 300155 VAL A, 300156 THR A, 300157 VAL A, 300040 THR B
Unique lining residues set - sidechains
300046 GLU A, 300061 ASN A, 300063 LYS A, 300003 LEU B, 300064 ILE A, 20 SER B, 18 ARG B, 300040 ARG A, 300091 SER A, 300041 PRO A, 300088 SER A, 300175 LEU A, 300180 TYR A, 300153 GLU A, 300172 PRO A, 300041 ASN B, 300182 LEU A, 300170 THR A, 300156 THR A,
Physicochemical properties of lining side-chains
Charge: 1 (3-2)
Hydropathy: -1.2
Hydrophobicity: -0.36
Polarity: 12.09
Mutability: 84
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 300046 GLU A, 300061 ASN A, 300063 LYS A, 300003 LEU B, 300098 PHE B 4.26 1.13 -1.5 -0.4 21.26 76 2 300046 GLU A, 300063 LYS A, 300003 LEU B, 300098 PHE B 4.28 2.22 -1 -0.3 25.73 67 3 300046 GLU A, 300063 LYS A, 300003 LEU B, 300064 ILE A 4.39 5.15 0.23 0.35 24.92 76 4 300046 GLU A, 300063 LYS A, 300003 LEU B, 20 SER B 5.72 6.18 -1.1 -0.35 25.3 80 5 300046 GLU A, 300063 LYS A, 300064 ILE A 2.91 11.16 -0.97 0.09 33.18 84 6 300046 GLU A, 300064 ILE A, 300063 LYS A 3.3 11.82 0.2 -0.04 17.8 90 7 300046 GLU A, 300064 ILE A, 300063 LYS A, 18 ARG B 3.62 14.5 -0.98 -0.14 26.35 87 8 300046 GLU A, 300064 ILE A, 18 ARG B 3.63 14.98 -1.17 0.08 34.01 87 9 300046 GLU A, 300064 ILE A, 18 ARG B, 300040 ARG A 3.53 18.3 -2 -0.04 38.51 86 10 300046 GLU A, 18 ARG B, 300040 ARG A 4 20.56 -4.17 -0.66 51.3 81 11 300046 GLU A, 300064 ILE A, 300040 ARG A 4.06 21.34 -1.17 0.08 34.01 87 12 300046 GLU A, 300040 ARG A 2.89 24.13 -4 -0.78 50.95 80 13 300040 ARG A -0.27 30.72 -4.5 -0.42 52 83 14 300040 ARG A, 300088 SER A 1.51 31.47 -2.45 -0.61 27.69 83 15 300040 ARG A, 300088 SER A, 300091 SER A 2.16 32.2 -1.77 -0.67 19.59 83 16 300040 ARG A, 300088 SER A, 300091 SER A 2.32 32.39 -1.9 -0.73 19.02 100 17 300040 ARG A, 300088 SER A, 300091 SER A, 300041 PRO A 2.48 32.62 -1.83 -0.57 14.66 86 18 300040 ARG A, 300088 SER A, 300091 SER A, 300041 PRO A 2.71 33.23 -1.73 -0.53 15.09 70 19 300040 ARG A, 300088 SER A, 300041 PRO A 3.01 35.82 -2.17 -0.44 18.99 70 20 300040 ARG A, 300041 PRO A, 300088 SER A 4.49 37.87 -2.3 -0.49 18.42 86 21 300041 PRO A, 300088 SER A 4.81 39.01 -1.2 -0.53 1.63 87 22 300091 SER A, 300041 PRO A, 300088 SER A 4.57 39.81 -1.07 -0.68 1.64 97 23 300091 SER A, 300041 PRO A, 300088 SER A, 300175 LEU A 4.53 40.44 0.15 -0.22 1.26 86 24 300041 PRO A, 300088 SER A, 300175 LEU A, 300180 TYR A 4.56 40.66 0.02 0.3 1.25 69 25 300091 SER A, 300088 SER A, 300175 LEU A, 300180 TYR A 4.34 40.99 0.23 0.08 1.27 84 26 300091 SER A, 300088 SER A, 300180 TYR A 4.07 41.15 -0.97 -0.28 1.65 94 27 300091 SER A, 300088 SER A 4.13 41.29 -0.8 -0.97 1.67 117 28 300091 SER A, 300088 SER A, 300180 TYR A 4.3 41.6 -0.97 -0.28 1.65 94 29 300091 SER A, 300088 SER A, 300175 LEU A, 300180 TYR A 4.4 42.11 0.23 0.08 1.27 84 30 300088 SER A, 300091 SER A, 300041 PRO A, 300175 LEU A, 300180 TYR A 4.35 42.78 -0.06 0.08 1.67 69 31 300041 PRO A, 300175 LEU A, 300180 TYR A 4.04 47.08 0.3 0.72 1.11 54 32 300041 PRO A, 300175 LEU A, 300180 TYR A, 300173 ALA A 4.46 47.42 0.13 0.34 1.68 54 33 300041 PRO A, 300175 LEU A, 300180 TYR A 4.37 48.05 0.3 0.72 1.11 54 34 300041 PRO A, 300175 LEU A, 300180 TYR A, 300173 ALA A 4.44 48.64 0.13 0.34 1.68 54 35 300041 PRO A, 300180 TYR A, 300173 ALA A 3.47 50.97 -1.1 0.07 2.19 54 36 300041 PRO A, 300180 TYR A, 300173 ALA A, 300153 GLU A 3.28 52.87 -1.7 -0.23 14.12 61 37 300041 PRO A, 300173 ALA A, 300153 GLU A, 300172 PRO A 3.35 55.01 -1.78 -0.53 14.11 64 38 300041 PRO A, 300153 GLU A, 300172 PRO A, 300041 ASN B 3.38 55.73 -2.55 -0.52 14.11 74 39 300041 PRO A, 300153 GLU A, 300172 PRO A, 300041 ASN B 3.47 56.22 -2.55 -0.52 14.11 74 40 300153 GLU A, 300172 PRO A, 300041 ASN B 3.11 56.87 -2.87 -0.67 18.29 79 41 300173 ALA A, 300153 GLU A, 300172 PRO A, 300041 ASN B 2.27 60.27 -2.25 -0.7 14.56 79 42 300173 ALA A, 300153 GLU A, 300041 ASN B, 300172 PRO A 2.44 60.7 -1.95 -0.88 15.01 90 43 300173 ALA A, 300153 GLU A, 300041 ASN B, 300172 PRO A, 300171 PHE A 2.62 61.04 -1.64 -0.86 12.68 90 44 300173 ALA A, 300153 GLU A, 300041 ASN B, 300172 PRO A, 300182 LEU A 2.74 61.28 -0.8 -0.47 12.03 78 45 300153 GLU A, 300041 ASN B, 300172 PRO A, 300171 PHE A, 300182 LEU A 2.62 61.48 -0.8 -0.47 12.03 78 46 300041 ASN B, 300172 PRO A, 300171 PHE A, 300182 LEU A 2.58 62.31 -0.13 -0.31 2.57 79 47 300041 ASN B, 300171 PHE A, 300182 LEU A 2.75 62.63 -0.03 -0.14 2.3 79 48 300041 ASN B, 300182 LEU A, 300170 THR A 2.76 62.87 -0.13 -0.13 1.72 88 49 300041 ASN B, 300182 LEU A, 300170 THR A, 300155 VAL A 2.61 64.04 -0.2 -0.3 2.14 88 50 300041 ASN B, 300170 THR A, 300155 VAL A 2.5 68.13 -1.53 -0.78 2.81 105 51 300041 ASN B, 300156 THR A, 300157 VAL A 3.14 68.5 -1.53 -0.78 2.81 105 52 300041 ASN B, 300170 THR A, 300156 THR A, 300157 VAL A 3.22 68.76 -1.33 -0.78 2.52 106 53 300041 ASN B, 300170 THR A, 300157 VAL A 3.23 68.84 -1.53 -0.78 2.81 105 54 300041 ASN B, 300170 THR A, 300155 VAL A 3.17 69.01 -1.53 -0.78 2.81 105 55 300041 ASN B, 300170 THR A, 300157 VAL A 3.23 69.23 -1.53 -0.78 2.81 105 56 300041 ASN B, 300170 THR A, 300156 THR A, 300157 VAL A 3.19 69.65 -1.33 -0.78 2.52 106 57 300170 THR A, 300156 THR A, 300157 VAL A 3.23 69.79 -0.6 -0.78 2.23 107 58 300041 ASN B, 300170 THR A, 300156 THR A, 300157 VAL A 3.29 70.38 -1.33 -0.78 2.52 106 59 300041 ASN B, 300156 THR A, 300157 VAL A 3.31 72.4 -1.53 -0.78 2.81 105 60 300041 ASN B, 300156 THR A, 300157 VAL A, 300040 THR B 3.79 72.99 -1.25 -0.79 2.95 105 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
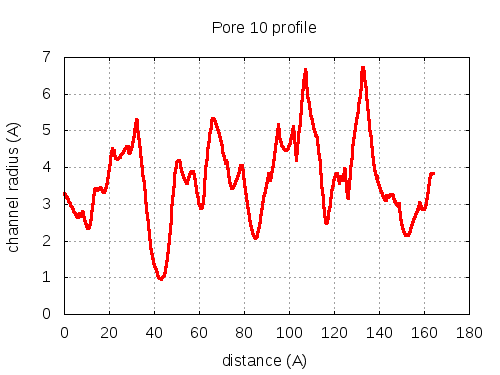
Unique lining residues set - all
100046 GLU A, 100061 ASN A, 100063 LYS A, 100003 LEU B, 100098 PHE B, 100064 ILE A, 300020 SER B, 100063 LYS A, 300018 ARG B, 100040 ARG A, 100088 SER A, 100091 SER A, 100041 PRO A, 100091 SER A, 100088 SER A, 100175 LEU A, 100180 TYR A, 100173 ALA A, 100153 GLU A, 100172 PRO A, 100041 ASN B, 100172 PRO A, 100171 PHE A, 100182 LEU A, 100170 THR A, 100155 VAL A, 100156 THR A, 100157 VAL A, 100040 THR B, 100168 VAL A, 100168 VAL A, 100169 LYS B, 100165 SER A, 100167 GLY A, 100169 HIS A, 100167 ASP B, 100166 SER A, 100166 SER A, 100170 ASP B, 100169 LYS B, 100140 MET A, 100138 ASN B, 100187 THR A, 100114 THR B, 100138 ASN A, 100139 SER A
Unique lining residues set - sidechains
100046 GLU A, 100061 ASN A, 100063 LYS A, 100003 LEU B, 100064 ILE A, 300020 SER B, 300018 ARG B, 100040 ARG A, 100041 PRO A, 100091 SER A, 100088 SER A, 100175 LEU A, 100180 TYR A, 100153 GLU A, 100172 PRO A, 100041 ASN B, 100182 LEU A, 100170 THR A, 100156 THR A, 100168 VAL A, 100169 LYS B, 100169 HIS A, 100167 ASP B, 100166 SER A, 100170 ASP B, 100140 MET A, 100138 ASN B, 100187 THR A, 100114 THR B, 100138 ASN A, 100139 SER A
Physicochemical properties of lining side-chains
Charge: 0 (4-4)
Hydropathy: -1.2
Hydrophobicity: -0.39
Polarity: 13.61
Mutability: 89
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 100046 GLU A, 100061 ASN A, 100063 LYS A, 100003 LEU B, 100098 PHE B 4.26 1.35 -1.5 -0.4 21.26 76 2 100046 GLU A, 100063 LYS A, 100003 LEU B, 100098 PHE B 4.45 2.62 -1 -0.3 25.73 67 3 100046 GLU A, 100063 LYS A, 100003 LEU B, 100064 ILE A 4.67 5.14 0.23 0.35 24.92 76 4 100046 GLU A, 100063 LYS A, 100003 LEU B, 300020 SER B 5.72 5.83 -1.1 -0.35 25.3 80 5 100046 GLU A, 100063 LYS A, 100003 LEU B, 100064 ILE A 5.65 6.5 0.23 0.35 24.92 76 6 100046 GLU A, 100063 LYS A, 100064 ILE A 2.93 11.08 -0.97 0.09 33.18 84 7 100046 GLU A, 100064 ILE A, 100063 LYS A 3.39 11.87 0.2 -0.04 17.8 90 8 100046 GLU A, 100064 ILE A, 100063 LYS A, 300018 ARG B 3.63 14.99 -0.98 -0.14 26.35 87 9 100046 GLU A, 100064 ILE A, 300018 ARG B, 100040 ARG A 3.53 18.28 -2 -0.04 38.51 86 10 100046 GLU A, 300018 ARG B, 100040 ARG A 4.05 20.58 -4.17 -0.66 51.3 81 11 100046 GLU A, 100064 ILE A, 100040 ARG A 4.2 21.65 -1.17 0.08 34.01 87 12 100046 GLU A, 100040 ARG A 2.18 24.04 -4 -0.78 50.95 80 13 100040 ARG A 0.49 30.43 -4.5 -0.42 52 83 14 100040 ARG A, 100088 SER A 1.51 31.39 -2.45 -0.61 27.69 83 15 100040 ARG A, 100088 SER A, 100091 SER A 1.91 32.22 -1.77 -0.67 19.59 83 16 100040 ARG A, 100088 SER A, 100041 PRO A, 100091 SER A 2.39 32.46 -1.83 -0.57 14.66 86 17 100040 ARG A, 100088 SER A, 100091 SER A, 100041 PRO A 2.56 33.15 -1.73 -0.53 15.09 70 18 100040 ARG A, 100088 SER A, 100041 PRO A 2.96 35.6 -2.17 -0.44 18.99 70 19 100040 ARG A, 100041 PRO A, 100088 SER A 4.34 37.84 -2.3 -0.49 18.42 86 20 100041 PRO A, 100088 SER A 4.73 39.22 -1.2 -0.53 1.63 87 21 100041 PRO A, 100091 SER A, 100088 SER A 4.66 39.69 -1.07 -0.68 1.64 97 22 100041 PRO A, 100091 SER A, 100088 SER A, 100175 LEU A 4.58 40.44 0.15 -0.22 1.26 86 23 100041 PRO A, 100088 SER A, 100175 LEU A, 100180 TYR A 4.45 40.68 0.02 0.3 1.25 69 24 100091 SER A, 100088 SER A, 100175 LEU A, 100180 TYR A 4.28 40.88 0.23 0.08 1.27 84 25 100091 SER A, 100088 SER A, 100180 TYR A 4.16 41.09 -0.97 -0.28 1.65 94 26 100091 SER A, 100088 SER A 4.32 41.28 -0.8 -0.97 1.67 117 27 100091 SER A, 100088 SER A, 100180 TYR A 4.5 41.71 -0.97 -0.28 1.65 94 28 100041 PRO A, 100088 SER A, 100175 LEU A, 100180 TYR A 4.55 42.42 0.02 0.3 1.25 69 29 100088 SER A, 100041 PRO A, 100091 SER A, 100175 LEU A, 100180 TYR A 4.42 43.32 -0.06 0.08 1.67 69 30 100041 PRO A, 100175 LEU A, 100180 TYR A 4.05 46.98 0.3 0.72 1.11 54 31 100041 PRO A, 100175 LEU A, 100180 TYR A, 100173 ALA A 4.45 47.41 0.13 0.34 1.68 54 32 100041 PRO A, 100175 LEU A, 100180 TYR A 4.38 47.99 0.3 0.72 1.11 54 33 100041 PRO A, 100175 LEU A, 100180 TYR A, 100173 ALA A 4.48 48.52 0.13 0.34 1.68 54 34 100041 PRO A, 100180 TYR A, 100173 ALA A 3.57 50.73 -1.1 0.07 2.19 54 35 100041 PRO A, 100180 TYR A, 100173 ALA A, 100153 GLU A 3.4 53.01 -1.7 -0.23 14.12 61 36 100041 PRO A, 100173 ALA A, 100153 GLU A, 100172 PRO A 3.39 55.14 -1.78 -0.53 14.11 64 37 100041 PRO A, 100153 GLU A, 100172 PRO A, 100041 ASN B 3.42 55.69 -2.55 -0.52 14.11 74 38 100041 PRO A, 100153 GLU A, 100172 PRO A, 100041 ASN B 3.36 56.31 -2.55 -0.52 14.11 74 39 100153 GLU A, 100172 PRO A, 100041 ASN B 3.24 56.7 -2.87 -0.67 18.29 79 40 100173 ALA A, 100153 GLU A, 100172 PRO A, 100041 ASN B 2.3 60.14 -2.25 -0.7 14.56 79 41 100173 ALA A, 100153 GLU A, 100041 ASN B, 100172 PRO A 2.4 60.66 -1.95 -0.88 15.01 90 42 100173 ALA A, 100153 GLU A, 100041 ASN B, 100172 PRO A, 100171 PHE A 2.55 61.05 -1.64 -0.86 12.68 90 43 100153 GLU A, 100041 ASN B, 100172 PRO A, 100171 PHE A, 100182 LEU A 2.68 61.45 -0.8 -0.47 12.03 78 44 100041 ASN B, 100172 PRO A, 100171 PHE A, 100182 LEU A 2.63 62.36 -0.13 -0.31 2.57 79 45 100041 ASN B, 100171 PHE A, 100182 LEU A 2.76 62.55 -0.03 -0.14 2.3 79 46 100041 ASN B, 100182 LEU A, 100170 THR A 2.79 62.83 -0.13 -0.13 1.72 88 47 100041 ASN B, 100182 LEU A, 100170 THR A, 100155 VAL A 2.64 64.49 -0.2 -0.3 2.14 88 48 100041 ASN B, 100170 THR A, 100155 VAL A 2.59 68.02 -1.53 -0.78 2.81 105 49 100041 ASN B, 100170 THR A, 100156 THR A, 100157 VAL A 3.16 68.21 -1.33 -0.78 2.52 106 50 100041 ASN B, 100156 THR A, 100157 VAL A 3.23 68.48 -1.53 -0.78 2.81 105 51 100041 ASN B, 100170 THR A, 100156 THR A, 100157 VAL A 3.19 68.63 -1.33 -0.78 2.52 106 52 100041 ASN B, 100170 THR A, 100157 VAL A 3.18 68.73 -1.53 -0.78 2.81 105 53 100041 ASN B, 100170 THR A, 100155 VAL A 3.18 68.87 -1.53 -0.78 2.81 105 54 100041 ASN B, 100170 THR A, 100157 VAL A 3.23 69.07 -1.53 -0.78 2.81 105 55 100041 ASN B, 100170 THR A, 100156 THR A, 100157 VAL A 3.27 69.53 -1.33 -0.78 2.52 106 56 100041 ASN B, 100170 THR A, 100157 VAL A 3.27 72.67 -1.53 -0.78 2.81 105 57 100041 ASN B, 100170 THR A, 100157 VAL A, 100040 THR B 4.12 73.42 -1.25 -0.79 2.95 105 58 100041 ASN B, 100170 THR A, 100157 VAL A, 100040 THR B, 100168 VAL A 4.55 73.56 -1.08 -0.79 3.04 105 59 100170 THR A, 100157 VAL A, 100040 THR B, 100168 VAL A 4.61 73.77 0.68 -0.31 2.14 102 60 100170 THR A, 100157 VAL A, 100040 THR B, 100168 VAL A, 100169 LYS B 4.56 73.92 -1.16 -0.72 12.26 89 61 100170 THR A, 100157 VAL A, 100168 VAL A, 100169 LYS B 4.45 74.65 -0.2 -0.21 13.67 92 62 100157 VAL A, 100168 VAL A, 100169 LYS B, 100165 SER A 4.08 75.44 -0.13 -0.22 14.1 85 63 100168 VAL A, 100169 LYS B, 100165 SER A 2.14 79.13 -0.03 -0.03 17.67 85 64 100168 VAL A, 100169 LYS B, 100165 SER A 1.44 81.38 -1.57 -0.67 18.75 72 65 100168 VAL A, 100169 LYS B, 100165 SER A, 100167 GLY A 1.54 82.53 -1.28 -0.7 14.91 72 66 100168 VAL A, 100169 LYS B, 100167 GLY A 1.71 83.69 -1.57 -0.67 18.75 72 67 100168 VAL A, 100169 LYS B, 100167 GLY A, 100169 HIS A 1.84 83.86 -1.98 -0.44 26.97 81 68 100168 VAL A, 100169 LYS B, 100167 GLY A, 100169 HIS A 1.9 84.07 -1.98 -0.44 26.97 81 69 100168 VAL A, 100169 LYS B, 100167 GLY A, 100169 HIS A 1.97 84.09 -1.98 -0.44 26.97 81 70 100168 VAL A, 100169 LYS B, 100167 GLY A, 100169 HIS A 1.85 84.48 -1.98 -0.44 26.97 81 71 100168 VAL A, 100167 GLY A, 100169 HIS A, 100167 ASP B 1.86 84.62 -1.88 -0.6 27.02 88 72 100169 LYS B, 100167 GLY A, 100169 HIS A, 100167 ASP B 1.83 85.21 -2.75 -0.5 38.55 83 73 100169 LYS B, 100167 GLY A, 100167 ASP B 1.64 86.43 -2.6 -0.75 34.19 79 74 100169 LYS B, 100167 GLY A 1.61 87.12 -2.15 -0.61 26.44 72 75 100169 LYS B, 100167 GLY A, 100166 SER A 1.68 88.64 -1.57 -0.67 18.75 72 76 100169 LYS B, 100167 GLY A, 100166 SER A 1.83 88.93 -1.7 -0.73 18.18 94 77 100169 LYS B, 100166 SER A 1.83 89.15 -2.35 -0.69 25.59 94 78 100169 LYS B, 100167 GLY A, 100166 SER A 1.91 92.26 -1.7 -0.73 18.18 94 79 100169 LYS B, 100167 GLY A, 100166 SER A, 100170 ASP B 3.47 93.64 -2.15 -0.81 26.06 91 80 100167 GLY A, 100166 SER A, 100170 ASP B, 100169 LYS B 4.21 94.07 -1.28 -0.9 14.53 101 81 100167 GLY A, 100166 SER A, 100170 ASP B 4.45 95.19 -1.57 -0.94 18.25 101 82 100167 GLY A, 100166 SER A, 100170 ASP B, 100140 MET A 5.04 96.01 -0.7 -0.45 14.05 98 83 100167 GLY A, 100170 ASP B, 100140 MET A 4.21 97.74 -0.67 -0.28 18.17 89 84 100167 GLY A, 100170 ASP B, 100140 MET A, 100138 ASN B 3.9 98.88 -1.38 -0.4 14.47 94 85 100170 ASP B, 100140 MET A, 100138 ASN B 3.94 100.39 -1.7 -0.27 18.17 94 86 100140 MET A, 100138 ASN B, 100187 THR A 3.51 102.45 -0.77 -0.18 2.16 101 87 100140 MET A, 100138 ASN B, 100187 THR A, 100114 THR B 2.77 104.12 -0.75 -0.33 2.03 102 88 100140 MET A, 100187 THR A, 100114 THR B 2.56 105.75 0.17 -0.18 1.58 102 89 100140 MET A, 100114 THR B 2.7 107.05 0.6 0.12 1.55 100 90 100140 MET A, 100114 THR B, 100138 ASN A 3.05 107.55 -0.77 -0.18 2.16 101 91 100140 MET A, 100114 THR B, 100138 ASN A, 100139 SER A 2.84 108.61 -0.78 -0.38 2.04 105 92 100140 MET A, 100114 THR B, 100138 ASN A 2.77 109.41 -0.77 -0.18 2.16 101 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
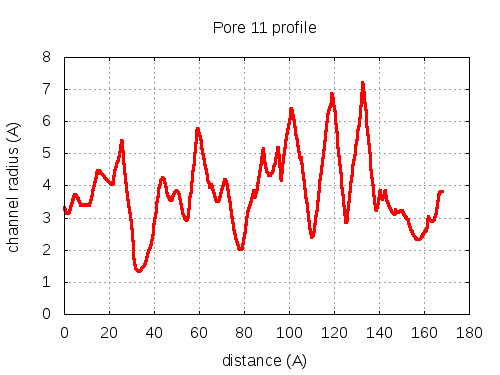
Unique lining residues set - all
46 GLU A, 61 ASN A, 63 LYS A, 3 LEU B, 98 PHE B, 64 ILE A, 200020 SER B, 63 LYS A, 200018 ARG B, 40 ARG A, 88 SER A, 91 SER A, 41 PRO A, 91 SER A, 88 SER A, 175 LEU A, 180 TYR A, 173 ALA A, 153 GLU A, 172 PRO A, 41 ASN B, 172 PRO A, 171 PHE A, 182 LEU A, 170 THR A, 155 VAL A, 156 THR A, 157 VAL A, 40 THR B, 168 VAL A, 168 VAL A, 169 LYS B, 165 SER A, 167 GLY A, 169 HIS A, 167 ASP B, 166 SER A, 166 SER A, 170 ASP B, 169 LYS B, 140 MET A, 138 ASN B, 187 THR A, 114 THR B, 138 ASN A, 139 SER A
Unique lining residues set - sidechains
46 GLU A, 61 ASN A, 63 LYS A, 3 LEU B, 64 ILE A, 200020 SER B, 200018 ARG B, 40 ARG A, 41 PRO A, 91 SER A, 88 SER A, 175 LEU A, 180 TYR A, 153 GLU A, 172 PRO A, 41 ASN B, 182 LEU A, 170 THR A, 156 THR A, 168 VAL A, 169 LYS B, 169 HIS A, 167 ASP B, 166 SER A, 170 ASP B, 140 MET A, 138 ASN B, 187 THR A, 114 THR B, 138 ASN A, 139 SER A
Physicochemical properties of lining side-chains
Charge: 0 (4-4)
Hydropathy: -1.2
Hydrophobicity: -0.39
Polarity: 13.61
Mutability: 89
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 46 GLU A, 61 ASN A, 63 LYS A, 3 LEU B, 98 PHE B 4.26 1.35 -1.5 -0.4 21.26 76 2 46 GLU A, 63 LYS A, 3 LEU B, 98 PHE B 4.45 2.62 -1 -0.3 25.73 67 3 46 GLU A, 63 LYS A, 3 LEU B, 64 ILE A 4.67 5.14 0.23 0.35 24.92 76 4 46 GLU A, 63 LYS A, 3 LEU B, 200020 SER B 5.72 5.83 -1.1 -0.35 25.3 80 5 46 GLU A, 63 LYS A, 3 LEU B, 64 ILE A 5.65 6.5 0.23 0.35 24.92 76 6 46 GLU A, 63 LYS A, 64 ILE A 2.93 11.08 -0.97 0.09 33.18 84 7 46 GLU A, 64 ILE A, 63 LYS A 3.39 11.87 0.2 -0.04 17.8 90 8 46 GLU A, 64 ILE A, 63 LYS A, 200018 ARG B 3.63 14.99 -0.98 -0.14 26.35 87 9 46 GLU A, 64 ILE A, 200018 ARG B, 40 ARG A 3.53 18.28 -2 -0.04 38.51 86 10 46 GLU A, 200018 ARG B, 40 ARG A 4.05 20.58 -4.17 -0.66 51.3 81 11 46 GLU A, 64 ILE A, 40 ARG A 4.2 21.65 -1.17 0.08 34.01 87 12 46 GLU A, 40 ARG A 2.18 24.04 -4 -0.78 50.95 80 13 40 ARG A 0.49 30.43 -4.5 -0.42 52 83 14 40 ARG A, 88 SER A 1.51 31.39 -2.45 -0.61 27.69 83 15 40 ARG A, 88 SER A, 91 SER A 1.91 32.22 -1.77 -0.67 19.59 83 16 40 ARG A, 88 SER A, 41 PRO A, 91 SER A 2.39 32.46 -1.83 -0.57 14.66 86 17 40 ARG A, 88 SER A, 91 SER A, 41 PRO A 2.56 33.15 -1.73 -0.53 15.09 70 18 40 ARG A, 88 SER A, 41 PRO A 2.96 35.6 -2.17 -0.44 18.99 70 19 40 ARG A, 41 PRO A, 88 SER A 4.34 37.84 -2.3 -0.49 18.42 86 20 41 PRO A, 88 SER A 4.73 39.22 -1.2 -0.53 1.63 87 21 41 PRO A, 91 SER A, 88 SER A 4.66 39.69 -1.07 -0.68 1.64 97 22 41 PRO A, 91 SER A, 88 SER A, 175 LEU A 4.58 40.44 0.15 -0.22 1.26 86 23 41 PRO A, 88 SER A, 175 LEU A, 180 TYR A 4.45 40.68 0.02 0.3 1.25 69 24 91 SER A, 88 SER A, 175 LEU A, 180 TYR A 4.28 40.88 0.23 0.08 1.27 84 25 91 SER A, 88 SER A, 180 TYR A 4.16 41.09 -0.97 -0.28 1.65 94 26 91 SER A, 88 SER A 4.32 41.28 -0.8 -0.97 1.67 117 27 91 SER A, 88 SER A, 180 TYR A 4.5 41.71 -0.97 -0.28 1.65 94 28 41 PRO A, 88 SER A, 175 LEU A, 180 TYR A 4.55 42.42 0.02 0.3 1.25 69 29 88 SER A, 41 PRO A, 91 SER A, 175 LEU A, 180 TYR A 4.42 43.32 -0.06 0.08 1.67 69 30 41 PRO A, 175 LEU A, 180 TYR A 4.05 46.98 0.3 0.72 1.11 54 31 41 PRO A, 175 LEU A, 180 TYR A, 173 ALA A 4.45 47.41 0.13 0.34 1.68 54 32 41 PRO A, 175 LEU A, 180 TYR A 4.38 47.99 0.3 0.72 1.11 54 33 41 PRO A, 175 LEU A, 180 TYR A, 173 ALA A 4.48 48.53 0.13 0.34 1.68 54 34 41 PRO A, 180 TYR A, 173 ALA A 3.57 50.73 -1.1 0.07 2.19 54 35 41 PRO A, 180 TYR A, 173 ALA A, 153 GLU A 3.4 53.01 -1.7 -0.23 14.12 61 36 41 PRO A, 173 ALA A, 153 GLU A, 172 PRO A 3.39 55.14 -1.78 -0.53 14.11 64 37 41 PRO A, 153 GLU A, 172 PRO A, 41 ASN B 3.42 55.69 -2.55 -0.52 14.11 74 38 41 PRO A, 153 GLU A, 172 PRO A, 41 ASN B 3.36 56.31 -2.55 -0.52 14.11 74 39 153 GLU A, 172 PRO A, 41 ASN B 3.24 56.7 -2.87 -0.67 18.29 79 40 173 ALA A, 153 GLU A, 172 PRO A, 41 ASN B 2.3 60.14 -2.25 -0.7 14.56 79 41 173 ALA A, 153 GLU A, 41 ASN B, 172 PRO A 2.4 60.66 -1.95 -0.88 15.01 90 42 173 ALA A, 153 GLU A, 41 ASN B, 172 PRO A, 171 PHE A 2.55 61.05 -1.64 -0.86 12.68 90 43 153 GLU A, 41 ASN B, 172 PRO A, 171 PHE A, 182 LEU A 2.68 61.45 -0.8 -0.47 12.03 78 44 41 ASN B, 172 PRO A, 171 PHE A, 182 LEU A 2.63 62.37 -0.13 -0.31 2.57 79 45 41 ASN B, 171 PHE A, 182 LEU A 2.76 62.55 -0.03 -0.14 2.3 79 46 41 ASN B, 182 LEU A, 170 THR A 2.79 62.83 -0.13 -0.13 1.72 88 47 41 ASN B, 182 LEU A, 170 THR A, 155 VAL A 2.64 64.49 -0.2 -0.3 2.14 88 48 41 ASN B, 170 THR A, 155 VAL A 2.59 68.02 -1.53 -0.78 2.81 105 49 41 ASN B, 170 THR A, 156 THR A, 157 VAL A 3.16 68.21 -1.33 -0.78 2.52 106 50 41 ASN B, 156 THR A, 157 VAL A 3.23 68.48 -1.53 -0.78 2.81 105 51 41 ASN B, 170 THR A, 156 THR A, 157 VAL A 3.19 68.64 -1.33 -0.78 2.52 106 52 41 ASN B, 170 THR A, 157 VAL A 3.18 68.73 -1.53 -0.78 2.81 105 53 41 ASN B, 170 THR A, 155 VAL A 3.18 68.87 -1.53 -0.78 2.81 105 54 41 ASN B, 170 THR A, 157 VAL A 3.23 69.08 -1.53 -0.78 2.81 105 55 41 ASN B, 170 THR A, 156 THR A, 157 VAL A 3.27 69.53 -1.33 -0.78 2.52 106 56 41 ASN B, 170 THR A, 157 VAL A 3.27 72.67 -1.53 -0.78 2.81 105 57 41 ASN B, 170 THR A, 157 VAL A, 40 THR B 4.12 73.42 -1.25 -0.79 2.95 105 58 41 ASN B, 170 THR A, 157 VAL A, 40 THR B, 168 VAL A 4.55 73.57 -1.08 -0.79 3.04 105 59 170 THR A, 157 VAL A, 40 THR B, 168 VAL A 4.61 73.77 0.68 -0.31 2.14 102 60 170 THR A, 157 VAL A, 40 THR B, 168 VAL A, 169 LYS B 4.56 73.92 -1.16 -0.72 12.26 89 61 170 THR A, 157 VAL A, 168 VAL A, 169 LYS B 4.45 74.65 -0.2 -0.21 13.67 92 62 157 VAL A, 168 VAL A, 169 LYS B, 165 SER A 4.08 75.44 -0.13 -0.22 14.1 85 63 168 VAL A, 169 LYS B, 165 SER A 2.14 79.14 -0.03 -0.03 17.67 85 64 168 VAL A, 169 LYS B, 165 SER A 1.44 81.38 -1.57 -0.67 18.75 72 65 168 VAL A, 169 LYS B, 165 SER A, 167 GLY A 1.54 82.53 -1.28 -0.7 14.91 72 66 168 VAL A, 169 LYS B, 167 GLY A 1.71 83.7 -1.57 -0.67 18.75 72 67 168 VAL A, 169 LYS B, 167 GLY A, 169 HIS A 1.84 83.86 -1.98 -0.44 26.97 81 68 168 VAL A, 169 LYS B, 167 GLY A, 169 HIS A 1.9 84.07 -1.98 -0.44 26.97 81 69 168 VAL A, 169 LYS B, 167 GLY A, 169 HIS A 1.97 84.09 -1.98 -0.44 26.97 81 70 168 VAL A, 169 LYS B, 167 GLY A, 169 HIS A 1.85 84.48 -1.98 -0.44 26.97 81 71 168 VAL A, 167 GLY A, 169 HIS A, 167 ASP B 1.86 84.62 -1.88 -0.6 27.02 88 72 169 LYS B, 167 GLY A, 169 HIS A, 167 ASP B 1.83 85.22 -2.75 -0.5 38.55 83 73 169 LYS B, 167 GLY A, 167 ASP B 1.64 86.43 -2.6 -0.75 34.19 79 74 169 LYS B, 167 GLY A 1.61 87.12 -2.15 -0.61 26.44 72 75 169 LYS B, 167 GLY A, 166 SER A 1.68 88.64 -1.57 -0.67 18.75 72 76 169 LYS B, 167 GLY A, 166 SER A 1.83 88.93 -1.7 -0.73 18.18 94 77 169 LYS B, 166 SER A 1.83 89.15 -2.35 -0.69 25.59 94 78 169 LYS B, 167 GLY A, 166 SER A 1.91 92.26 -1.7 -0.73 18.18 94 79 169 LYS B, 167 GLY A, 166 SER A, 170 ASP B 3.47 93.64 -2.15 -0.81 26.06 91 80 167 GLY A, 166 SER A, 170 ASP B, 169 LYS B 4.21 94.07 -1.28 -0.9 14.53 101 81 167 GLY A, 166 SER A, 170 ASP B 4.45 95.2 -1.57 -0.94 18.25 101 82 167 GLY A, 166 SER A, 170 ASP B, 140 MET A 5.04 96.01 -0.7 -0.45 14.05 98 83 167 GLY A, 170 ASP B, 140 MET A 4.21 97.74 -0.67 -0.28 18.17 89 84 167 GLY A, 170 ASP B, 140 MET A, 138 ASN B 3.9 98.88 -1.38 -0.4 14.47 94 85 170 ASP B, 140 MET A, 138 ASN B 3.94 100.39 -1.7 -0.27 18.17 94 86 140 MET A, 138 ASN B, 187 THR A 3.51 102.45 -0.77 -0.18 2.16 101 87 140 MET A, 138 ASN B, 187 THR A, 114 THR B 2.77 104.13 -0.75 -0.33 2.03 102 88 140 MET A, 187 THR A, 114 THR B 2.56 105.75 0.17 -0.18 1.58 102 89 140 MET A, 114 THR B 2.7 107.05 0.6 0.12 1.55 100 90 140 MET A, 114 THR B, 138 ASN A 3.05 107.55 -0.77 -0.18 2.16 101 91 140 MET A, 114 THR B, 138 ASN A, 139 SER A 2.84 108.61 -0.78 -0.38 2.04 105 92 140 MET A, 114 THR B, 138 ASN A 2.77 109.41 -0.77 -0.18 2.16 101 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
Unique lining residues set - all
200046 GLU A, 200061 ASN A, 200063 LYS A, 200003 LEU B, 200098 PHE B, 200064 ILE A, 100020 SER B, 200063 LYS A, 100018 ARG B, 200040 ARG A, 200088 SER A, 200091 SER A, 200041 PRO A, 200091 SER A, 200088 SER A, 200175 LEU A, 200180 TYR A, 200173 ALA A, 200153 GLU A, 200172 PRO A, 200041 ASN B, 200172 PRO A, 200171 PHE A, 200182 LEU A, 200170 THR A, 200155 VAL A, 200156 THR A, 200157 VAL A, 200040 THR B, 200168 VAL A, 200168 VAL A, 200169 LYS B, 200165 SER A, 200167 GLY A, 200169 HIS A, 200167 ASP B, 200166 SER A, 200166 SER A, 200170 ASP B, 200169 LYS B, 200140 MET A, 200138 ASN B, 200187 THR A, 200114 THR B, 200138 ASN A, 200139 SER A
Unique lining residues set - sidechains
200046 GLU A, 200061 ASN A, 200063 LYS A, 200003 LEU B, 200064 ILE A, 100020 SER B, 100018 ARG B, 200040 ARG A, 200041 PRO A, 200091 SER A, 200088 SER A, 200175 LEU A, 200180 TYR A, 200153 GLU A, 200172 PRO A, 200041 ASN B, 200182 LEU A, 200170 THR A, 200156 THR A, 200168 VAL A, 200169 LYS B, 200169 HIS A, 200167 ASP B, 200166 SER A, 200170 ASP B, 200140 MET A, 200138 ASN B, 200187 THR A, 200114 THR B, 200138 ASN A, 200139 SER A
Physicochemical properties of lining side-chains
Charge: 0 (4-4)
Hydropathy: -1.2
Hydrophobicity: -0.39
Polarity: 13.61
Mutability: 89
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 200046 GLU A, 200061 ASN A, 200063 LYS A, 200003 LEU B, 200098 PHE B 4.26 1.35 -1.5 -0.4 21.26 76 2 200046 GLU A, 200063 LYS A, 200003 LEU B, 200098 PHE B 4.45 2.62 -1 -0.3 25.73 67 3 200046 GLU A, 200063 LYS A, 200003 LEU B, 200064 ILE A 4.67 5.14 0.23 0.35 24.92 76 4 200046 GLU A, 200063 LYS A, 200003 LEU B, 100020 SER B 5.72 5.83 -1.1 -0.35 25.3 80 5 200046 GLU A, 200063 LYS A, 200003 LEU B, 200064 ILE A 5.65 6.5 0.23 0.35 24.92 76 6 200046 GLU A, 200063 LYS A, 200064 ILE A 2.93 11.08 -0.97 0.09 33.18 84 7 200046 GLU A, 200064 ILE A, 200063 LYS A 3.39 11.87 0.2 -0.04 17.8 90 8 200046 GLU A, 200064 ILE A, 200063 LYS A, 100018 ARG B 3.63 14.99 -0.98 -0.14 26.35 87 9 200046 GLU A, 200064 ILE A, 100018 ARG B, 200040 ARG A 3.53 18.28 -2 -0.04 38.51 86 10 200046 GLU A, 100018 ARG B, 200040 ARG A 4.05 20.58 -4.17 -0.66 51.3 81 11 200046 GLU A, 200064 ILE A, 200040 ARG A 4.2 21.65 -1.17 0.08 34.01 87 12 200046 GLU A, 200040 ARG A 2.18 24.04 -4 -0.78 50.95 80 13 200040 ARG A 0.49 30.43 -4.5 -0.42 52 83 14 200040 ARG A, 200088 SER A 1.51 31.39 -2.45 -0.61 27.69 83 15 200040 ARG A, 200088 SER A, 200091 SER A 1.91 32.22 -1.77 -0.67 19.59 83 16 200040 ARG A, 200088 SER A, 200041 PRO A, 200091 SER A 2.39 32.46 -1.83 -0.57 14.66 86 17 200040 ARG A, 200088 SER A, 200091 SER A, 200041 PRO A 2.56 33.15 -1.73 -0.53 15.09 70 18 200040 ARG A, 200088 SER A, 200041 PRO A 2.96 35.6 -2.17 -0.44 18.99 70 19 200040 ARG A, 200041 PRO A, 200088 SER A 4.34 37.84 -2.3 -0.49 18.42 86 20 200041 PRO A, 200088 SER A 4.73 39.22 -1.2 -0.53 1.63 87 21 200041 PRO A, 200091 SER A, 200088 SER A 4.66 39.69 -1.07 -0.68 1.64 97 22 200041 PRO A, 200091 SER A, 200088 SER A, 200175 LEU A 4.58 40.44 0.15 -0.22 1.26 86 23 200041 PRO A, 200088 SER A, 200175 LEU A, 200180 TYR A 4.45 40.68 0.02 0.3 1.25 69 24 200091 SER A, 200088 SER A, 200175 LEU A, 200180 TYR A 4.28 40.88 0.23 0.08 1.27 84 25 200091 SER A, 200088 SER A, 200180 TYR A 4.16 41.09 -0.97 -0.28 1.65 94 26 200091 SER A, 200088 SER A 4.32 41.28 -0.8 -0.97 1.67 117 27 200091 SER A, 200088 SER A, 200180 TYR A 4.5 41.71 -0.97 -0.28 1.65 94 28 200041 PRO A, 200088 SER A, 200175 LEU A, 200180 TYR A 4.55 42.42 0.02 0.3 1.25 69 29 200088 SER A, 200041 PRO A, 200091 SER A, 200175 LEU A, 200180 TYR A 4.42 43.32 -0.06 0.08 1.67 69 30 200041 PRO A, 200175 LEU A, 200180 TYR A 4.05 46.98 0.3 0.72 1.11 54 31 200041 PRO A, 200175 LEU A, 200180 TYR A, 200173 ALA A 4.45 47.41 0.13 0.34 1.68 54 32 200041 PRO A, 200175 LEU A, 200180 TYR A 4.38 47.99 0.3 0.72 1.11 54 33 200041 PRO A, 200175 LEU A, 200180 TYR A, 200173 ALA A 4.48 48.52 0.13 0.34 1.68 54 34 200041 PRO A, 200180 TYR A, 200173 ALA A 3.57 50.73 -1.1 0.07 2.19 54 35 200041 PRO A, 200180 TYR A, 200173 ALA A, 200153 GLU A 3.4 53.01 -1.7 -0.23 14.12 61 36 200041 PRO A, 200173 ALA A, 200153 GLU A, 200172 PRO A 3.39 55.14 -1.78 -0.53 14.11 64 37 200041 PRO A, 200153 GLU A, 200172 PRO A, 200041 ASN B 3.42 55.69 -2.55 -0.52 14.11 74 38 200041 PRO A, 200153 GLU A, 200172 PRO A, 200041 ASN B 3.36 56.31 -2.55 -0.52 14.11 74 39 200153 GLU A, 200172 PRO A, 200041 ASN B 3.24 56.7 -2.87 -0.67 18.29 79 40 200173 ALA A, 200153 GLU A, 200172 PRO A, 200041 ASN B 2.3 60.14 -2.25 -0.7 14.56 79 41 200173 ALA A, 200153 GLU A, 200041 ASN B, 200172 PRO A 2.4 60.66 -1.95 -0.88 15.01 90 42 200173 ALA A, 200153 GLU A, 200041 ASN B, 200172 PRO A, 200171 PHE A 2.55 61.05 -1.64 -0.86 12.68 90 43 200153 GLU A, 200041 ASN B, 200172 PRO A, 200171 PHE A, 200182 LEU A 2.68 61.45 -0.8 -0.47 12.03 78 44 200041 ASN B, 200172 PRO A, 200171 PHE A, 200182 LEU A 2.63 62.36 -0.13 -0.31 2.57 79 45 200041 ASN B, 200171 PHE A, 200182 LEU A 2.76 62.55 -0.03 -0.14 2.3 79 46 200041 ASN B, 200182 LEU A, 200170 THR A 2.79 62.83 -0.13 -0.13 1.72 88 47 200041 ASN B, 200182 LEU A, 200170 THR A, 200155 VAL A 2.64 64.49 -0.2 -0.3 2.14 88 48 200041 ASN B, 200170 THR A, 200155 VAL A 2.59 68.02 -1.53 -0.78 2.81 105 49 200041 ASN B, 200170 THR A, 200156 THR A, 200157 VAL A 3.16 68.21 -1.33 -0.78 2.52 106 50 200041 ASN B, 200156 THR A, 200157 VAL A 3.23 68.48 -1.53 -0.78 2.81 105 51 200041 ASN B, 200170 THR A, 200156 THR A, 200157 VAL A 3.19 68.63 -1.33 -0.78 2.52 106 52 200041 ASN B, 200170 THR A, 200157 VAL A 3.18 68.73 -1.53 -0.78 2.81 105 53 200041 ASN B, 200170 THR A, 200155 VAL A 3.18 68.87 -1.53 -0.78 2.81 105 54 200041 ASN B, 200170 THR A, 200157 VAL A 3.23 69.08 -1.53 -0.78 2.81 105 55 200041 ASN B, 200170 THR A, 200156 THR A, 200157 VAL A 3.27 69.53 -1.33 -0.78 2.52 106 56 200041 ASN B, 200170 THR A, 200157 VAL A 3.27 72.67 -1.53 -0.78 2.81 105 57 200041 ASN B, 200170 THR A, 200157 VAL A, 200040 THR B 4.12 73.42 -1.25 -0.79 2.95 105 58 200041 ASN B, 200170 THR A, 200157 VAL A, 200040 THR B, 200168 VAL A 4.55 73.56 -1.08 -0.79 3.04 105 59 200170 THR A, 200157 VAL A, 200040 THR B, 200168 VAL A 4.61 73.77 0.68 -0.31 2.14 102 60 200170 THR A, 200157 VAL A, 200040 THR B, 200168 VAL A, 200169 LYS B 4.56 73.92 -1.16 -0.72 12.26 89 61 200170 THR A, 200157 VAL A, 200168 VAL A, 200169 LYS B 4.45 74.65 -0.2 -0.21 13.67 92 62 200157 VAL A, 200168 VAL A, 200169 LYS B, 200165 SER A 4.08 75.44 -0.13 -0.22 14.1 85 63 200168 VAL A, 200169 LYS B, 200165 SER A 2.14 79.14 -0.03 -0.03 17.67 85 64 200168 VAL A, 200169 LYS B, 200165 SER A 1.44 81.38 -1.57 -0.67 18.75 72 65 200168 VAL A, 200169 LYS B, 200165 SER A, 200167 GLY A 1.54 82.53 -1.28 -0.7 14.91 72 66 200168 VAL A, 200169 LYS B, 200167 GLY A 1.71 83.69 -1.57 -0.67 18.75 72 67 200168 VAL A, 200169 LYS B, 200167 GLY A, 200169 HIS A 1.84 83.86 -1.98 -0.44 26.97 81 68 200168 VAL A, 200169 LYS B, 200167 GLY A, 200169 HIS A 1.9 84.07 -1.98 -0.44 26.97 81 69 200168 VAL A, 200169 LYS B, 200167 GLY A, 200169 HIS A 1.97 84.09 -1.98 -0.44 26.97 81 70 200168 VAL A, 200169 LYS B, 200167 GLY A, 200169 HIS A 1.85 84.48 -1.98 -0.44 26.97 81 71 200168 VAL A, 200167 GLY A, 200169 HIS A, 200167 ASP B 1.86 84.62 -1.88 -0.6 27.02 88 72 200169 LYS B, 200167 GLY A, 200169 HIS A, 200167 ASP B 1.83 85.21 -2.75 -0.5 38.55 83 73 200169 LYS B, 200167 GLY A, 200167 ASP B 1.64 86.43 -2.6 -0.75 34.19 79 74 200169 LYS B, 200167 GLY A 1.61 87.12 -2.15 -0.61 26.44 72 75 200169 LYS B, 200167 GLY A, 200166 SER A 1.68 88.64 -1.57 -0.67 18.75 72 76 200169 LYS B, 200167 GLY A, 200166 SER A 1.83 88.93 -1.7 -0.73 18.18 94 77 200169 LYS B, 200166 SER A 1.83 89.15 -2.35 -0.69 25.59 94 78 200169 LYS B, 200167 GLY A, 200166 SER A 1.91 92.26 -1.7 -0.73 18.18 94 79 200169 LYS B, 200167 GLY A, 200166 SER A, 200170 ASP B 3.47 93.64 -2.15 -0.81 26.06 91 80 200167 GLY A, 200166 SER A, 200170 ASP B, 200169 LYS B 4.21 94.07 -1.28 -0.9 14.53 101 81 200167 GLY A, 200166 SER A, 200170 ASP B 4.45 95.2 -1.57 -0.94 18.25 101 82 200167 GLY A, 200166 SER A, 200170 ASP B, 200140 MET A 5.04 96.01 -0.7 -0.45 14.05 98 83 200167 GLY A, 200170 ASP B, 200140 MET A 4.21 97.74 -0.67 -0.28 18.17 89 84 200167 GLY A, 200170 ASP B, 200140 MET A, 200138 ASN B 3.9 98.88 -1.38 -0.4 14.47 94 85 200170 ASP B, 200140 MET A, 200138 ASN B 3.94 100.39 -1.7 -0.27 18.17 94 86 200140 MET A, 200138 ASN B, 200187 THR A 3.51 102.45 -0.77 -0.18 2.16 101 87 200140 MET A, 200138 ASN B, 200187 THR A, 200114 THR B 2.77 104.12 -0.75 -0.33 2.03 102 88 200140 MET A, 200187 THR A, 200114 THR B 2.56 105.75 0.17 -0.18 1.58 102 89 200140 MET A, 200114 THR B 2.7 107.05 0.6 0.12 1.55 100 90 200140 MET A, 200114 THR B, 200138 ASN A 3.05 107.55 -0.77 -0.18 2.16 101 91 200140 MET A, 200114 THR B, 200138 ASN A, 200139 SER A 2.84 108.61 -0.78 -0.38 2.04 105 92 200140 MET A, 200114 THR B, 200138 ASN A 2.77 109.41 -0.77 -0.18 2.16 101 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
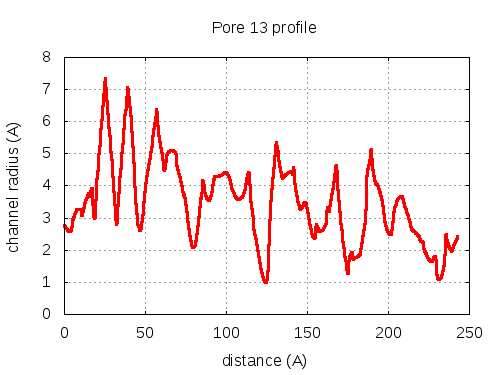
Unique lining residues set - all
200042 GLY A, 200040 THR B, 200085 ASN B, 200165 ASP B, 200041 ASN B, 200172 PRO A, 200041 PRO A, 200153 GLU A, 200173 ALA A, 200180 TYR A, 200175 LEU A, 200088 SER A, 200091 SER A, 200088 SER A, 200040 ARG A, 200091 SER A, 200043 HIS A, 200046 GLU A, 100018 ARG B, 200064 ILE A, 200063 LYS A, 200063 LYS A, 100020 SER B, 200003 LEU B, 200098 PHE B, 200061 ASN A, 200097 THR B, 200001 ASP B, 100072 THR B, 100070 ASP B, 200001 ASP B, 200026 SER B, 100024 ARG B, 200026 SER B, 100069 THR B, 100026 SER B, 100027 GLN B, 100028 SER B, 100028 SER B, 200027 GLN B, 100027 GLN B, 200028 SER B, 27 GLN B, 28 SER B, 300027 GLN B, 300028 SER B, 200028 SER B, 200093 ARG B, 200082 TYR C, 100093 ARG B, 100058 GLN C, 100030 GLY B, 100092 ASN B, 200084 VAL C, 100064 ARG C, 100032 ASP B, 100031 THR B, 100031 THR B, 200084 VAL C, 100050 TYR B, 100053 GLU B, 200085 THR C, 200085 THR C, 200054 ALA C, 200053 GLY C, 200057 ARG A
Unique lining residues set - sidechains
200040 THR B, 200085 ASN B, 200165 ASP B, 200041 ASN B, 200172 PRO A, 200041 PRO A, 200153 GLU A, 200180 TYR A, 200175 LEU A, 200091 SER A, 200088 SER A, 200040 ARG A, 200043 HIS A, 200046 GLU A, 100018 ARG B, 200064 ILE A, 200063 LYS A, 100020 SER B, 200003 LEU B, 200061 ASN A, 200097 THR B, 200001 ASP B, 100072 THR B, 100070 ASP B, 200026 SER B, 100024 ARG B, 100069 THR B, 100028 SER B, 200027 GLN B, 100027 GLN B, 27 GLN B, 28 SER B, 300027 GLN B, 200028 SER B, 200093 ARG B, 200082 TYR C, 100093 ARG B, 100058 GLN C, 100092 ASN B, 200084 VAL C, 100064 ARG C, 100032 ASP B, 100031 THR B, 100050 TYR B, 100053 GLU B, 200085 THR C, 200054 ALA C, 200057 ARG A
Physicochemical properties of lining side-chains
Charge: 1 (8-7)
Hydropathy: -1.4
Hydrophobicity: -0.42
Polarity: 15.82
Mutability: 89
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 200042 GLY A, 200040 THR B, 200085 ASN B, 200165 ASP B 3.3 0.13 -2.03 -0.85 14.53 99 2 200042 GLY A, 200040 THR B, 200165 ASP B 3.16 1.85 -1.53 -0.87 18.25 96 3 200042 GLY A, 200040 THR B, 200165 ASP B, 200041 ASN B 3.19 3.92 -2.03 -0.85 14.53 99 4 200042 GLY A, 200040 THR B, 200165 ASP B, 200041 ASN B, 200172 PRO A 3.67 4.14 -1.94 -0.69 11.94 88 5 200042 GLY A, 200165 ASP B, 200041 ASN B, 200172 PRO A 3.68 4.73 -2.25 -0.68 14.51 82 6 200042 GLY A, 200041 ASN B, 200172 PRO A, 200041 PRO A 3.69 5.03 -1.78 -0.44 2.48 73 7 200042 GLY A, 200041 ASN B, 200172 PRO A, 200041 PRO A 3.71 5.26 -1.78 -0.44 2.48 73 8 200042 GLY A, 200041 ASN B, 200172 PRO A, 200041 PRO A 3.63 6.24 -1.78 -0.44 2.48 73 9 200041 ASN B, 200172 PRO A, 200041 PRO A 3.58 6.55 -2.23 -0.32 2.18 73 10 200041 ASN B, 200172 PRO A, 200041 PRO A, 200153 GLU A 3.48 7.07 -2.55 -0.52 14.11 74 11 200041 ASN B, 200172 PRO A, 200041 PRO A, 200153 GLU A 3.42 7.91 -2.55 -0.52 14.11 74 12 200172 PRO A, 200041 PRO A, 200153 GLU A, 200173 ALA A 3.43 9.94 -1.78 -0.53 14.11 64 13 200041 PRO A, 200153 GLU A, 200173 ALA A, 200180 TYR A 3.28 12.14 -1.7 -0.23 14.12 61 14 200041 PRO A, 200173 ALA A, 200180 TYR A 3.46 14.12 -1.1 0.07 2.19 54 15 200041 PRO A, 200173 ALA A, 200180 TYR A, 200175 LEU A 4.43 14.65 0.13 0.34 1.68 54 16 200041 PRO A, 200180 TYR A, 200175 LEU A 4.38 15.3 0.3 0.72 1.11 54 17 200041 PRO A, 200173 ALA A, 200180 TYR A, 200175 LEU A 4.43 15.84 0.13 0.34 1.68 54 18 200041 PRO A, 200180 TYR A, 200175 LEU A 4.09 20.47 0.3 0.72 1.11 54 19 200041 PRO A, 200180 TYR A, 200175 LEU A, 200088 SER A, 200091 SER A 4.51 21.03 -0.06 0.08 1.67 69 20 200180 TYR A, 200175 LEU A, 200091 SER A, 200088 SER A 4.57 21.35 0.23 0.08 1.27 84 21 200180 TYR A, 200091 SER A, 200088 SER A 4.53 21.48 -0.97 -0.28 1.65 94 22 200091 SER A, 200088 SER A 4.43 21.55 -0.8 -0.97 1.67 117 23 200180 TYR A, 200091 SER A, 200088 SER A 4.24 21.87 -0.97 -0.28 1.65 94 24 200180 TYR A, 200175 LEU A, 200091 SER A, 200088 SER A 4.24 22.09 0.23 0.08 1.27 84 25 200041 PRO A, 200180 TYR A, 200175 LEU A, 200088 SER A 4.3 22.75 0.02 0.3 1.25 69 26 200041 PRO A, 200175 LEU A, 200091 SER A, 200088 SER A 4.52 23.17 0.15 -0.22 1.26 86 27 200041 PRO A, 200091 SER A, 200088 SER A 4.64 24.08 -1.07 -0.68 1.64 97 28 200041 PRO A, 200088 SER A 4.87 25.13 -1.2 -0.53 1.63 87 29 200041 PRO A, 200088 SER A, 200040 ARG A 4.4 27.48 -2.3 -0.49 18.42 86 30 200041 PRO A, 200088 SER A, 200040 ARG A 3.1 29.6 -2.17 -0.44 18.99 70 31 200041 PRO A, 200088 SER A, 200040 ARG A, 200091 SER A 2.78 30.24 -1.73 -0.53 15.09 70 32 200088 SER A, 200040 ARG A, 200091 SER A 1.76 31.21 -1.77 -0.67 19.59 83 33 200088 SER A, 200040 ARG A 0.42 33.11 -2.45 -0.61 27.69 83 34 200040 ARG A 0.03 38.61 -4.5 -0.42 52 83 35 200040 ARG A, 200043 HIS A 2.46 39.68 -3.85 -0.08 51.8 87 36 200040 ARG A, 200046 GLU A 3.24 43.59 -4 -0.78 50.95 80 37 200040 ARG A, 200046 GLU A, 100018 ARG B 3.9 46.1 -4.17 -0.66 51.3 81 38 200040 ARG A, 200046 GLU A, 100018 ARG B, 200064 ILE A 3.5 49.53 -2 -0.04 38.51 86 39 200046 GLU A, 100018 ARG B, 200064 ILE A 3.7 50.08 -1.17 0.08 34.01 87 40 200046 GLU A, 100018 ARG B, 200064 ILE A, 200063 LYS A 3.5 53.31 -0.98 -0.14 26.35 87 41 200046 GLU A, 200064 ILE A, 200063 LYS A 2.87 58.21 -0.97 0.09 33.18 84 42 200046 GLU A, 200064 ILE A, 200063 LYS A, 100020 SER B 5.6 58.61 -0.93 -0.18 25.3 92 43 200046 GLU A, 200063 LYS A, 100020 SER B, 200003 LEU B 5.96 59.5 -1.1 -0.35 25.3 80 44 200046 GLU A, 200064 ILE A, 200063 LYS A, 200003 LEU B 4.63 62.51 0.23 0.35 24.92 76 45 200046 GLU A, 200063 LYS A, 200003 LEU B, 200098 PHE B 4.19 63.47 -1 -0.3 25.73 67 46 200046 GLU A, 200063 LYS A, 200003 LEU B, 200098 PHE B, 200061 ASN A 3.84 64.6 -1.5 -0.4 21.26 76 47 200046 GLU A, 200063 LYS A, 200098 PHE B, 200061 ASN A, 200097 THR B 3.86 64.74 -2.4 -0.78 21.56 90 48 200046 GLU A, 200098 PHE B, 200061 ASN A, 200097 THR B 3.94 64.93 -2.03 -0.87 14.58 96 49 200046 GLU A, 200063 LYS A, 200098 PHE B, 200061 ASN A, 200097 THR B 4.15 65.13 -2.4 -0.78 21.56 90 50 200063 LYS A, 200003 LEU B, 200098 PHE B, 200061 ASN A, 200097 THR B 4.08 65.93 -0.94 -0.32 11.61 84 51 200063 LYS A, 200003 LEU B, 200098 PHE B, 200097 THR B 3.91 66.31 -0.3 -0.21 13.67 77 52 200063 LYS A, 200003 LEU B, 200061 ASN A, 200097 THR B 3.72 66.71 -1.08 -0.2 13.67 84 53 200063 LYS A, 200003 LEU B, 200097 THR B, 200001 ASP B 3.41 68.56 -1.08 -0.27 25.25 79 54 200063 LYS A, 200003 LEU B, 200001 ASP B 3.52 70.23 -1.2 -0.1 33.11 70 55 200063 LYS A, 200003 LEU B, 200001 ASP B, 100072 THR B 3.99 71.21 -1.08 -0.27 25.25 79 56 200063 LYS A, 200003 LEU B, 200001 ASP B, 100072 THR B, 100070 ASP B 4.15 71.6 -1.56 -0.42 30.14 81 57 200003 LEU B, 200001 ASP B, 100072 THR B, 100070 ASP B 4.01 72.37 -0.98 -0.43 25.3 83 58 200003 LEU B, 100072 THR B, 100070 ASP B, 200001 ASP B 3.94 72.52 -0.2 -0.37 13.72 82 59 200003 LEU B, 100070 ASP B, 200001 ASP B 3.11 74.46 -0.03 -0.23 17.74 70 60 200003 LEU B, 100070 ASP B 2.2 77.35 0.15 0.05 24.92 70 61 200003 LEU B, 100070 ASP B, 200026 SER B 2.16 80.12 -0.17 -0.29 17.17 85 62 200003 LEU B, 100070 ASP B, 200026 SER B, 100024 ARG B 2.48 82.17 -1.25 -0.32 25.88 85 63 100070 ASP B, 200026 SER B, 100024 ARG B 3.29 83.71 -2.93 -0.81 34.46 95 64 100070 ASP B, 100024 ARG B, 200026 SER B 3.64 84.79 -2.8 -0.75 35.03 84 65 100070 ASP B, 100024 ARG B, 200026 SER B, 100069 THR B 3.73 86.2 -2.28 -0.76 26.69 92 66 100024 ARG B, 200026 SER B, 100069 THR B 4.27 87.7 -1.87 -0.66 19.01 95 67 100024 ARG B, 200026 SER B, 100069 THR B, 100026 SER B 4.87 88.73 -1.5 -0.7 15.11 95 68 200026 SER B, 100069 THR B, 100026 SER B 4.45 91.48 -0.5 -0.79 2.81 107 69 200026 SER B, 100069 THR B, 100026 SER B, 100027 GLN B 4.46 92.37 -0.48 -0.79 2.95 107 70 200026 SER B, 100069 THR B, 100026 SER B, 100027 GLN B, 100028 SER B 4.54 94.55 -0.46 -0.79 3.04 107 71 200026 SER B, 100027 GLN B, 100028 SER B, 200027 GLN B 5.13 94.83 -1.28 -0.92 2.99 100 72 200026 SER B, 100069 THR B, 100028 SER B, 200027 GLN B 4.17 97.73 -1.35 -0.91 2.56 102 73 200026 SER B, 100028 SER B, 200027 GLN B 5.13 98.36 -1.57 -0.96 2.86 100 74 100027 GLN B, 100028 SER B, 200027 GLN B 5.59 98.97 -1.57 -0.96 2.86 100 75 100028 SER B, 200027 GLN B, 100027 GLN B, 200028 SER B 6.05 99.91 -2.05 -0.99 3.03 95 76 27 GLN B, 28 SER B, 300027 GLN B, 300028 SER B 6.14 101.09 -2.05 -0.99 3.03 95 77 100028 SER B, 200027 GLN B, 200028 SER B 5.03 104.5 -1.7 -1.01 2.29 106 78 100028 SER B, 200027 GLN B, 200028 SER B, 200093 ARG B 4.85 104.83 -2.4 -0.87 14.72 100 79 200027 GLN B, 200028 SER B, 200093 ARG B 4.8 105.38 -2.93 -0.83 19.07 94 80 100028 SER B, 200027 GLN B, 200028 SER B, 200093 ARG B 4.65 105.81 -2.4 -0.87 14.72 100 81 100028 SER B, 200027 GLN B, 200028 SER B, 200093 ARG B 4.41 106.4 -2.3 -0.82 15.15 94 82 100028 SER B, 200027 GLN B, 200093 ARG B 2.44 111.27 -2.93 -0.83 19.07 94 83 100028 SER B, 200093 ARG B 2.92 111.96 -2.65 -0.7 26.84 100 84 100028 SER B, 200093 ARG B, 200082 TYR C 3.2 113.48 -2.2 -0.09 18.43 83 85 100028 SER B, 200093 ARG B, 200082 TYR C, 100093 ARG B 3.91 113.8 -2.78 -0.18 26.82 83 86 100028 SER B, 200082 TYR C, 100093 ARG B 4 114.09 -2.2 -0.09 18.43 83 87 100028 SER B, 200082 TYR C, 100093 ARG B, 100058 GLN C 3.83 115.21 -2.53 -0.35 14.7 83 88 100028 SER B, 200082 TYR C, 100058 GLN C 3.17 117.88 -1.87 -0.32 2.27 83 89 100028 SER B, 200082 TYR C, 100058 GLN C, 100030 GLY B 3.11 118.77 -1.5 -0.44 2.55 83 90 200082 TYR C, 100058 GLN C, 100030 GLY B, 100092 ASN B 3.14 120.71 -2.18 -0.39 2.98 79 91 200082 TYR C, 100030 GLY B, 100092 ASN B 3.25 121.04 -1.73 -0.15 2.79 77 92 200082 TYR C, 100030 GLY B, 100092 ASN B, 200084 VAL C 3.32 121.8 -0.25 0.17 2.13 84 93 200082 TYR C, 100030 GLY B, 100092 ASN B, 200084 VAL C, 100064 ARG C 3.26 122.07 -1.1 0.05 12.1 83 94 100030 GLY B, 100092 ASN B, 200084 VAL C, 100064 ARG C, 100032 ASP B 3.05 123.4 -1.54 -0.38 21.72 92 95 100030 GLY B, 200084 VAL C, 100064 ARG C, 100032 ASP B 3 124.33 -1.05 -0.28 26.3 89 96 100030 GLY B, 200084 VAL C, 100064 ARG C 2.97 124.47 -0.23 -0.03 18.5 90 97 100030 GLY B, 200084 VAL C, 100064 ARG C, 100031 THR B 2.94 124.57 -0.35 -0.22 14.29 96 98 100030 GLY B, 100064 ARG C, 100031 THR B 2.94 124.59 -1.87 -0.66 19.01 95 99 100030 GLY B, 100064 ARG C, 100032 ASP B, 100031 THR B 2.96 124.65 -2.2 -0.77 27.12 84 100 100030 GLY B, 100064 ARG C, 100031 THR B 3.01 124.72 -1.87 -0.66 19.01 95 101 200084 VAL C, 100064 ARG C, 100032 ASP B, 100031 THR B 2.91 124.98 -1.13 -0.28 25.87 93 102 200084 VAL C, 100064 ARG C, 100031 THR B 2.82 125.19 -0.33 -0.02 17.93 96 103 200084 VAL C, 100064 ARG C, 100032 ASP B, 100031 THR B 2.71 125.46 -1.13 -0.28 25.87 93 104 200084 VAL C, 100064 ARG C, 100031 THR B 2.49 126.29 -0.33 -0.02 17.93 96 105 200084 VAL C, 100064 ARG C, 100032 ASP B, 100031 THR B 2.38 128.2 -1.13 -0.28 25.87 93 106 100064 ARG C, 100032 ASP B, 100031 THR B, 200084 VAL C 2.52 128.87 -2.28 -0.76 26.69 92 107 100064 ARG C, 100032 ASP B, 100031 THR B, 200084 VAL C, 100050 TYR B 2.67 129.96 -2.08 -0.38 21.67 81 108 100064 ARG C, 100031 THR B, 200084 VAL C, 100050 TYR B 2.91 130.5 -1.73 -0.22 14.66 80 109 100064 ARG C, 100031 THR B, 100050 TYR B 2.88 130.6 -2.17 -0.03 18.42 80 110 100031 THR B, 100050 TYR B, 100053 GLU B 2.82 130.68 -1.83 -0.27 17.72 78 111 100031 THR B, 200084 VAL C, 100050 TYR B, 100053 GLU B 2.79 132.21 -1.48 -0.4 14.14 78 112 100031 THR B, 200084 VAL C, 100050 TYR B, 100053 GLU B, 200085 THR C 3.11 132.63 -1.26 -0.48 11.99 78 113 100031 THR B, 200084 VAL C, 100053 GLU B, 200085 THR C 3.09 133.52 -1.33 -0.87 14.15 97 114 100031 THR B, 200084 VAL C, 100053 GLU B, 200085 THR C, 200054 ALA C 2.91 134.72 -0.7 -0.69 11.32 97 115 100031 THR B, 100053 GLU B, 200085 THR C, 200054 ALA C 2.87 135.28 -0.78 -0.67 13.31 97 116 100053 GLU B, 200085 THR C, 200054 ALA C 2.89 136.51 -0.8 -0.63 17.19 94 117 100053 GLU B, 200085 THR C, 200054 ALA C, 200053 GLY C 3.07 138.38 -0.7 -0.67 13.74 94 118 100053 GLU B, 200085 THR C, 200054 ALA C, 200053 GLY C, 200057 ARG A 3.55 138.96 -1.46 -0.62 21.39 91 119 100053 GLU B, 200085 THR C, 200053 GLY C, 200057 ARG A 3.77 139.41 -2.28 -0.78 26.74 89 120 100053 GLU B, 200053 GLY C, 200057 ARG A 3.83 140.31 -2.8 -0.79 35.09 80 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
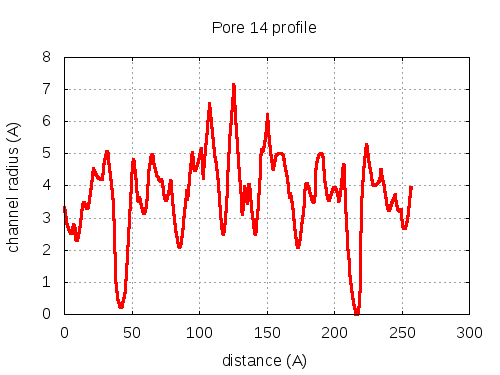
Unique lining residues set - all
100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B, 100155 VAL A, 100182 LEU A, 100171 PHE A, 100172 PRO A, 100153 GLU A, 100173 ALA A, 100172 PRO A, 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A, 100091 SER A, 100088 SER A, 100040 ARG A, 100091 SER A, 100043 HIS A, 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A, 100063 LYS A, 300020 SER B, 100003 LEU B, 100098 PHE B, 100061 ASN A, 100097 THR B, 100001 ASP B, 300072 THR B, 300070 ASP B, 100001 ASP B, 100026 SER B, 300024 ARG B, 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B, 300028 SER B, 300028 SER B, 100027 GLN B, 300027 GLN B, 100028 SER B, 100028 SER B, 27 GLN B, 28 SER B, 200027 GLN B, 200028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 300058 GLN C, 300030 GLY B, 300092 ASN B, 100084 VAL C, 300064 ARG C, 300032 ASP B, 300031 THR B, 300031 THR B, 100084 VAL C, 300050 TYR B, 300053 GLU B, 100085 THR C, 100054 ALA C, 100053 GLY C, 100057 ARG A
Unique lining residues set - sidechains
100156 THR A, 100170 THR A, 100041 ASN B, 100182 LEU A, 100153 GLU A, 100172 PRO A, 100041 PRO A, 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A, 100040 ARG A, 100043 HIS A, 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A, 300020 SER B, 100003 LEU B, 100061 ASN A, 100097 THR B, 100001 ASP B, 300072 THR B, 300070 ASP B, 100026 SER B, 300024 ARG B, 300069 THR B, 300028 SER B, 100027 GLN B, 300027 GLN B, 100028 SER B, 27 GLN B, 200027 GLN B, 200028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 300058 GLN C, 300092 ASN B, 100084 VAL C, 300064 ARG C, 300032 ASP B, 300031 THR B, 300050 TYR B, 300053 GLU B, 100085 THR C, 100054 ALA C, 100057 ARG A
Physicochemical properties of lining side-chains
Charge: 2 (8-6)
Hydropathy: -1.4
Hydrophobicity: -0.44
Polarity: 14.93
Mutability: 88
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B 3.28 0.25 -1.33 -0.78 2.52 106 2 100157 VAL A, 100170 THR A, 100041 ASN B 3.23 0.44 -1.53 -0.78 2.81 105 3 100170 THR A, 100041 ASN B, 100155 VAL A 3.21 0.71 -1.53 -0.78 2.81 105 4 100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B 3.22 1.01 -1.33 -0.78 2.52 106 5 100156 THR A, 100157 VAL A, 100041 ASN B 3.04 1.41 -1.53 -0.78 2.81 105 6 100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B, 100155 VAL A 2.92 1.92 -1.14 -0.78 2.69 106 7 100170 THR A, 100041 ASN B, 100155 VAL A 2.62 5.6 -1.53 -0.78 2.81 105 8 100170 THR A, 100041 ASN B, 100155 VAL A, 100182 LEU A 2.67 6.6 -0.2 -0.3 2.14 88 9 100170 THR A, 100041 ASN B, 100182 LEU A 2.76 6.95 -0.13 -0.13 1.72 88 10 100041 ASN B, 100182 LEU A, 100171 PHE A 2.75 7.13 -0.03 -0.14 2.3 79 11 100041 ASN B, 100182 LEU A, 100171 PHE A, 100172 PRO A 2.66 7.98 -0.13 -0.31 2.57 79 12 100041 ASN B, 100182 LEU A, 100171 PHE A, 100172 PRO A, 100153 GLU A 2.51 8.51 -0.8 -0.47 12.03 78 13 100041 ASN B, 100182 LEU A, 100172 PRO A, 100153 GLU A, 100173 ALA A 2.43 8.98 -0.8 -0.47 12.03 78 14 100041 ASN B, 100172 PRO A, 100153 GLU A, 100173 ALA A 2.36 9.54 -1.95 -0.88 15.01 90 15 100041 ASN B, 100153 GLU A, 100173 ALA A, 100172 PRO A 2.33 12.81 -2.25 -0.7 14.56 79 16 100041 ASN B, 100153 GLU A, 100172 PRO A 3.11 13.46 -2.87 -0.67 18.29 79 17 100041 ASN B, 100153 GLU A, 100172 PRO A, 100041 PRO A 3.47 13.99 -2.55 -0.52 14.11 74 18 100041 ASN B, 100153 GLU A, 100172 PRO A, 100041 PRO A 3.52 14.61 -2.55 -0.52 14.11 74 19 100153 GLU A, 100173 ALA A, 100172 PRO A, 100041 PRO A 3.37 16.81 -1.78 -0.53 14.11 64 20 100153 GLU A, 100173 ALA A, 100041 PRO A, 100180 TYR A 3.32 18.64 -1.7 -0.23 14.12 61 21 100173 ALA A, 100041 PRO A, 100180 TYR A 3.52 21.08 -1.1 0.07 2.19 54 22 100173 ALA A, 100041 PRO A, 100180 TYR A, 100175 LEU A 4.41 21.42 0.13 0.34 1.68 54 23 100041 PRO A, 100180 TYR A, 100175 LEU A 4.39 22.11 0.3 0.72 1.11 54 24 100173 ALA A, 100041 PRO A, 100180 TYR A, 100175 LEU A 4.48 22.49 0.13 0.34 1.68 54 25 100041 PRO A, 100180 TYR A, 100175 LEU A 4.02 27.24 0.3 0.72 1.11 54 26 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A, 100091 SER A 4.06 27.82 -0.06 0.08 1.67 69 27 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A 4.13 28.13 0.23 0.08 1.27 84 28 100180 TYR A, 100091 SER A, 100088 SER A 4.21 28.25 -0.97 -0.28 1.65 94 29 100091 SER A, 100088 SER A 4.29 28.34 -0.8 -0.97 1.67 117 30 100180 TYR A, 100091 SER A, 100088 SER A 4.37 28.5 -0.97 -0.28 1.65 94 31 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A 4.44 28.96 0.23 0.08 1.27 84 32 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A 4.52 29.3 0.02 0.3 1.25 69 33 100041 PRO A, 100175 LEU A, 100091 SER A, 100088 SER A 4.54 29.73 0.15 -0.22 1.26 86 34 100041 PRO A, 100091 SER A, 100088 SER A 4.57 30.68 -1.07 -0.68 1.64 97 35 100041 PRO A, 100088 SER A 4.75 31.84 -1.2 -0.53 1.63 87 36 100041 PRO A, 100088 SER A, 100040 ARG A 4.42 34.37 -2.3 -0.49 18.42 86 37 100041 PRO A, 100088 SER A, 100040 ARG A 2.94 36.52 -2.17 -0.44 18.99 70 38 100041 PRO A, 100088 SER A, 100040 ARG A, 100091 SER A 2.78 36.86 -1.73 -0.53 15.09 70 39 100088 SER A, 100040 ARG A, 100091 SER A 1.14 38.5 -1.77 -0.67 19.59 83 40 100088 SER A, 100040 ARG A 0.6 39.61 -2.45 -0.61 27.69 83 41 100040 ARG A 0.31 45.39 -4.5 -0.42 52 83 42 100040 ARG A, 100043 HIS A 2.56 46.51 -3.85 -0.08 51.8 87 43 100040 ARG A, 100046 GLU A 3.28 50.56 -4 -0.78 50.95 80 44 100040 ARG A, 100046 GLU A, 300018 ARG B 3.82 52.63 -4.17 -0.66 51.3 81 45 100040 ARG A, 100046 GLU A, 300018 ARG B, 100064 ILE A 3.58 56.39 -2 -0.04 38.51 86 46 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A 3.37 59.68 -0.98 -0.14 26.35 87 47 100046 GLU A, 100064 ILE A, 100063 LYS A 3.24 60.46 0.2 -0.04 17.8 90 48 100046 GLU A, 100064 ILE A, 100063 LYS A 3.01 64.93 -0.97 0.09 33.18 84 49 100046 GLU A, 100064 ILE A, 100063 LYS A, 300020 SER B 5.66 65.36 -0.93 -0.18 25.3 92 50 100046 GLU A, 100063 LYS A, 300020 SER B, 100003 LEU B 5.69 66.34 -1.1 -0.35 25.3 80 51 100046 GLU A, 100064 ILE A, 100063 LYS A, 100003 LEU B 4.41 69.56 0.23 0.35 24.92 76 52 100046 GLU A, 100063 LYS A, 100003 LEU B, 100098 PHE B 4.13 70.51 -1 -0.3 25.73 67 53 100046 GLU A, 100063 LYS A, 100003 LEU B, 100098 PHE B, 100061 ASN A 3.98 71.14 -1.5 -0.4 21.26 76 54 100046 GLU A, 100063 LYS A, 100098 PHE B, 100061 ASN A, 100097 THR B 3.93 71.43 -2.4 -0.78 21.56 90 55 100046 GLU A, 100098 PHE B, 100061 ASN A, 100097 THR B 3.96 71.79 -2.03 -0.87 14.58 96 56 100046 GLU A, 100063 LYS A, 100098 PHE B, 100061 ASN A, 100097 THR B 4.19 72.01 -2.4 -0.78 21.56 90 57 100063 LYS A, 100003 LEU B, 100098 PHE B, 100061 ASN A, 100097 THR B 4.11 72.59 -0.94 -0.32 11.61 84 58 100063 LYS A, 100003 LEU B, 100098 PHE B, 100097 THR B 3.95 72.99 -0.3 -0.21 13.67 77 59 100063 LYS A, 100003 LEU B, 100061 ASN A, 100097 THR B 3.75 73.42 -1.08 -0.2 13.67 84 60 100063 LYS A, 100003 LEU B, 100097 THR B, 100001 ASP B 3.41 75.38 -1.08 -0.27 25.25 79 61 100063 LYS A, 100003 LEU B, 100001 ASP B 3.5 77.12 -1.2 -0.1 33.11 70 62 100063 LYS A, 100003 LEU B, 100001 ASP B, 300072 THR B 3.9 78.04 -1.08 -0.27 25.25 79 63 100063 LYS A, 100003 LEU B, 100001 ASP B, 300072 THR B, 300070 ASP B 4.14 78.24 -1.56 -0.42 30.14 81 64 100003 LEU B, 100001 ASP B, 300072 THR B, 300070 ASP B 3.97 79.12 -0.98 -0.43 25.3 83 65 100003 LEU B, 300072 THR B, 300070 ASP B, 100001 ASP B 3.92 79.28 -0.2 -0.37 13.72 82 66 100003 LEU B, 300070 ASP B, 100001 ASP B 2.93 81.61 -0.03 -0.23 17.74 70 67 100003 LEU B, 300070 ASP B 2.21 84.21 0.15 0.05 24.92 70 68 100003 LEU B, 300070 ASP B, 100026 SER B 2.16 87.12 -0.17 -0.29 17.17 85 69 100003 LEU B, 300070 ASP B, 100026 SER B, 300024 ARG B 2.6 88.83 -1.25 -0.32 25.88 85 70 300070 ASP B, 100026 SER B, 300024 ARG B 3.23 90.45 -2.93 -0.81 34.46 95 71 300070 ASP B, 300024 ARG B, 100026 SER B 3.64 91.4 -2.8 -0.75 35.03 84 72 300070 ASP B, 300024 ARG B, 100026 SER B, 300069 THR B 3.7 93.41 -2.28 -0.76 26.69 92 73 300024 ARG B, 100026 SER B, 300069 THR B 4.41 94.42 -1.87 -0.66 19.01 95 74 300024 ARG B, 100026 SER B, 300069 THR B, 300026 SER B 4.91 95.58 -1.5 -0.7 15.11 95 75 100026 SER B, 300069 THR B, 300026 SER B 4.38 98.7 -0.5 -0.79 2.81 107 76 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B 4.41 99.61 -0.48 -0.79 2.95 107 77 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B, 300028 SER B 4.56 101.23 -0.46 -0.79 3.04 107 78 100026 SER B, 300069 THR B, 300027 GLN B, 300028 SER B 5.15 101.39 -0.58 -0.84 2.52 112 79 100026 SER B, 300027 GLN B, 300028 SER B, 100027 GLN B 5.19 101.54 -1.28 -0.92 2.99 100 80 100026 SER B, 300069 THR B, 300028 SER B, 100027 GLN B 4.16 104.78 -1.35 -0.91 2.56 102 81 300027 GLN B, 300028 SER B, 100027 GLN B 5.29 105.45 -1.57 -0.96 2.86 100 82 300028 SER B, 100027 GLN B, 300027 GLN B 5.59 106.05 -2.6 -1.06 2.91 95 83 300028 SER B, 100027 GLN B, 300027 GLN B, 100028 SER B 5.88 106.53 -2.05 -0.99 3.03 95 84 300028 SER B, 100027 GLN B, 100028 SER B 6.13 106.84 -1.7 -1.01 2.29 106 85 27 GLN B, 28 SER B, 200027 GLN B, 200028 SER B 6.36 107.83 -2.05 -0.99 3.03 95 86 300028 SER B, 100027 GLN B, 100028 SER B 4.84 111.33 -1.7 -1.01 2.29 106 87 300028 SER B, 100027 GLN B, 100028 SER B, 100093 ARG B 4.78 111.61 -2.4 -0.87 14.72 100 88 100027 GLN B, 100028 SER B, 100093 ARG B 4.86 112.02 -2.93 -0.83 19.07 94 89 300028 SER B, 100027 GLN B, 100028 SER B, 100093 ARG B 4.69 112.99 -2.4 -0.87 14.72 100 90 300028 SER B, 100027 GLN B, 100093 ARG B 2.42 118.16 -2.93 -0.83 19.07 94 91 300028 SER B, 100093 ARG B 2.91 118.88 -2.65 -0.7 26.84 100 92 300028 SER B, 100093 ARG B, 100082 TYR C 3.23 120.36 -2.2 -0.09 18.43 83 93 300028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B 4.01 120.68 -2.78 -0.18 26.82 83 94 300028 SER B, 100082 TYR C, 300093 ARG B, 300058 GLN C 3.73 122.31 -2.53 -0.35 14.7 83 95 300028 SER B, 100082 TYR C, 300058 GLN C 3.21 124.59 -1.87 -0.32 2.27 83 96 300028 SER B, 100082 TYR C, 300058 GLN C, 300030 GLY B 3.12 125.31 -1.5 -0.44 2.55 83 97 100082 TYR C, 300058 GLN C, 300030 GLY B, 300092 ASN B 3.11 127.4 -2.18 -0.39 2.98 79 98 100082 TYR C, 300030 GLY B, 300092 ASN B 3.22 127.75 -1.73 -0.15 2.79 77 99 100082 TYR C, 300030 GLY B, 300092 ASN B, 100084 VAL C 3.23 128.41 -0.25 0.17 2.13 84 100 100082 TYR C, 300030 GLY B, 300092 ASN B, 100084 VAL C, 300064 ARG C 3.24 128.68 -1.1 0.05 12.1 83 101 300030 GLY B, 300092 ASN B, 100084 VAL C, 300064 ARG C, 300032 ASP B 3.11 129.97 -1.54 -0.38 21.72 92 102 300030 GLY B, 100084 VAL C, 300064 ARG C, 300032 ASP B 2.96 131.02 -1.05 -0.28 26.3 89 103 300030 GLY B, 100084 VAL C, 300064 ARG C 2.94 131.19 -0.23 -0.03 18.5 90 104 300030 GLY B, 100084 VAL C, 300064 ARG C, 300031 THR B 2.94 131.3 -0.35 -0.22 14.29 96 105 300030 GLY B, 300064 ARG C, 300031 THR B 2.96 131.32 -1.87 -0.66 19.01 95 106 300030 GLY B, 300064 ARG C, 300032 ASP B, 300031 THR B 2.98 131.35 -2.2 -0.77 27.12 84 107 300030 GLY B, 300064 ARG C, 300032 ASP B, 300031 THR B 3.02 131.39 -2.28 -0.76 26.69 92 108 300030 GLY B, 300064 ARG C, 300031 THR B 3 131.48 -1.87 -0.66 19.01 95 109 100084 VAL C, 300064 ARG C, 300032 ASP B, 300031 THR B 2.95 131.61 -1.13 -0.28 25.87 93 110 100084 VAL C, 300064 ARG C, 300031 THR B 2.76 132.05 -0.33 -0.02 17.93 96 111 100084 VAL C, 300064 ARG C, 300032 ASP B, 300031 THR B 2.65 132.39 -1.13 -0.28 25.87 93 112 100084 VAL C, 300064 ARG C, 300031 THR B 2.55 132.84 -0.33 -0.02 17.93 96 113 100084 VAL C, 300064 ARG C, 300032 ASP B, 300031 THR B 2.4 134.81 -1.13 -0.28 25.87 93 114 300064 ARG C, 300032 ASP B, 300031 THR B, 100084 VAL C 2.42 136.17 -2.28 -0.76 26.69 92 115 300064 ARG C, 300032 ASP B, 300031 THR B, 100084 VAL C, 300050 TYR B 2.58 136.69 -2.08 -0.38 21.67 81 116 300064 ARG C, 300031 THR B, 100084 VAL C, 300050 TYR B 2.68 137.24 -1.73 -0.22 14.66 80 117 300064 ARG C, 300031 THR B, 300050 TYR B 2.84 137.34 -2.17 -0.03 18.42 80 118 300031 THR B, 300050 TYR B, 300053 GLU B 2.88 137.43 -1.83 -0.27 17.72 78 119 300031 THR B, 100084 VAL C, 300050 TYR B, 300053 GLU B 2.92 139.15 -1.48 -0.4 14.14 78 120 300031 THR B, 100084 VAL C, 300053 GLU B, 100085 THR C 3.09 140.19 -1.33 -0.87 14.15 97 121 300031 THR B, 100084 VAL C, 300053 GLU B, 100085 THR C, 100054 ALA C 3 141.49 -0.7 -0.69 11.32 97 122 300031 THR B, 300053 GLU B, 100085 THR C, 100054 ALA C 3 142.09 -0.78 -0.67 13.31 97 123 300053 GLU B, 100085 THR C, 100054 ALA C 2.99 143.38 -0.8 -0.63 17.19 94 124 300053 GLU B, 100085 THR C, 100054 ALA C, 100053 GLY C 3.04 145 -0.7 -0.67 13.74 94 125 300053 GLU B, 100085 THR C, 100054 ALA C, 100053 GLY C, 100057 ARG A 3.5 145.64 -1.46 -0.62 21.39 91 126 300053 GLU B, 100085 THR C, 100053 GLY C, 100057 ARG A 3.79 146.09 -2.28 -0.78 26.74 89 127 300053 GLU B, 100053 GLY C, 100057 ARG A 3.83 147.03 -2.8 -0.79 35.09 80 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
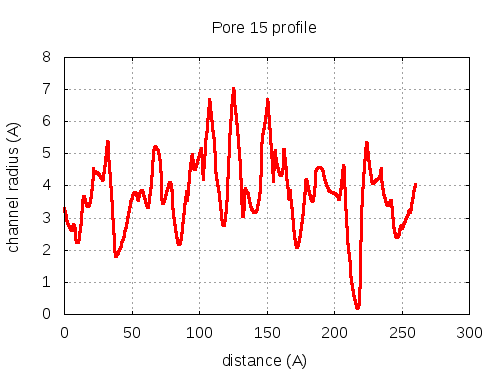
Unique lining residues set - all
300046 GLU A, 300061 ASN A, 300063 LYS A, 300003 LEU B, 300098 PHE B, 300064 ILE A, 20 SER B, 300063 LYS A, 18 ARG B, 300040 ARG A, 300088 SER A, 300091 SER A, 300091 SER A, 300041 PRO A, 300088 SER A, 300175 LEU A, 300180 TYR A, 300173 ALA A, 300153 GLU A, 300172 PRO A, 300041 ASN B, 300172 PRO A, 300182 LEU A, 300171 PHE A, 300170 THR A, 300155 VAL A, 300156 THR A, 300157 VAL A, 300040 THR B, 300168 VAL A, 300168 VAL A, 300169 LYS B, 300165 SER A, 300167 GLY A, 300169 HIS A, 300167 ASP B, 300166 SER A, 300166 SER A, 300170 ASP B, 300169 LYS B, 300140 MET A, 300138 ASN B, 300187 THR A, 300114 THR B, 300138 ASN A, 300139 SER A, 300113 PRO B, 300207 LYS B, 300115 VAL B, 300136 GLN A, 300116 SER B, 300140 MET A, 300135 ALA A, 300117 ILE B, 300116 SER B, 300117 ILE B, 300208 SER B, 300132 GLY A, 300209 PHE B, 300119 PRO B, 300210 ASN B, 300133 SER A, 300218 ARG A, 300211 ARG B, 300186 TYR B, 300211 ARG B, 300125 LEU B
Unique lining residues set - sidechains
300046 GLU A, 300061 ASN A, 300063 LYS A, 300003 LEU B, 300064 ILE A, 20 SER B, 18 ARG B, 300040 ARG A, 300091 SER A, 300041 PRO A, 300088 SER A, 300175 LEU A, 300180 TYR A, 300153 GLU A, 300172 PRO A, 300041 ASN B, 300182 LEU A, 300170 THR A, 300156 THR A, 300168 VAL A, 300169 LYS B, 300169 HIS A, 300167 ASP B, 300166 SER A, 300170 ASP B, 300140 MET A, 300138 ASN B, 300187 THR A, 300114 THR B, 300138 ASN A, 300139 SER A, 300207 LYS B, 300116 SER B, 300135 ALA A, 300117 ILE B, 300209 PHE B, 300119 PRO B, 300218 ARG A, 300186 TYR B, 300211 ARG B, 300125 LEU B
Physicochemical properties of lining side-chains
Charge: 3 (7-4)
Hydropathy: -1
Hydrophobicity: -0.37
Polarity: 11.83
Mutability: 86
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 300046 GLU A, 300061 ASN A, 300063 LYS A, 300003 LEU B, 300098 PHE B 4.26 1.42 -1.5 -0.4 21.26 76 2 300046 GLU A, 300063 LYS A, 300003 LEU B, 300098 PHE B 4.73 2.74 -1 -0.3 25.73 67 3 300046 GLU A, 300063 LYS A, 300003 LEU B, 300064 ILE A 5.15 5.23 0.23 0.35 24.92 76 4 300046 GLU A, 300063 LYS A, 300003 LEU B, 20 SER B 5.38 6.05 -1.1 -0.35 25.3 80 5 300046 GLU A, 300063 LYS A, 300064 ILE A 2.92 10.86 -0.97 0.09 33.18 84 6 300046 GLU A, 300064 ILE A, 300063 LYS A 3.05 11.69 0.2 -0.04 17.8 90 7 300046 GLU A, 300064 ILE A, 300063 LYS A, 18 ARG B 3.31 15.01 -0.98 -0.14 26.35 87 8 300046 GLU A, 300064 ILE A, 18 ARG B, 300040 ARG A 3.53 18 -2 -0.04 38.51 86 9 300046 GLU A, 18 ARG B, 300040 ARG A 3.92 20.52 -4.17 -0.66 51.3 81 10 300046 GLU A, 300064 ILE A, 300040 ARG A 4.05 21.59 -1.17 0.08 34.01 87 11 300046 GLU A, 300040 ARG A 2.98 24.09 -4 -0.78 50.95 80 12 300040 ARG A 1.23 30.7 -4.5 -0.42 52 83 13 300040 ARG A, 300088 SER A 1.25 31.59 -2.45 -0.61 27.69 83 14 300040 ARG A, 300088 SER A, 300091 SER A 1.51 32.05 -1.77 -0.67 19.59 83 15 300040 ARG A, 300088 SER A, 300091 SER A 1.91 32.31 -1.9 -0.73 19.02 100 16 300040 ARG A, 300088 SER A, 300091 SER A, 300041 PRO A 2.35 32.59 -1.83 -0.57 14.66 86 17 300040 ARG A, 300088 SER A, 300091 SER A, 300041 PRO A 2.74 33.41 -1.73 -0.53 15.09 70 18 300040 ARG A, 300088 SER A, 300041 PRO A 3.23 36.07 -2.17 -0.44 18.99 70 19 300040 ARG A, 300041 PRO A, 300088 SER A 4.55 37.86 -2.3 -0.49 18.42 86 20 300041 PRO A, 300088 SER A 4.79 39.33 -1.2 -0.53 1.63 87 21 300091 SER A, 300041 PRO A, 300088 SER A 4.69 39.81 -1.07 -0.68 1.64 97 22 300091 SER A, 300041 PRO A, 300088 SER A, 300175 LEU A 4.6 40.23 0.15 -0.22 1.26 86 23 300041 PRO A, 300088 SER A, 300175 LEU A, 300180 TYR A 4.5 40.53 0.02 0.3 1.25 69 24 300091 SER A, 300088 SER A, 300175 LEU A, 300180 TYR A 4.27 40.95 0.23 0.08 1.27 84 25 300091 SER A, 300088 SER A, 300180 TYR A 4.02 41.52 -0.97 -0.28 1.65 94 26 300091 SER A, 300088 SER A, 300175 LEU A, 300180 TYR A 4.01 42.17 0.23 0.08 1.27 84 27 300088 SER A, 300091 SER A, 300041 PRO A, 300175 LEU A, 300180 TYR A 4.02 43.08 -0.06 0.08 1.67 69 28 300041 PRO A, 300175 LEU A, 300180 TYR A 4.07 46.98 0.3 0.72 1.11 54 29 300041 PRO A, 300175 LEU A, 300180 TYR A, 300173 ALA A 4.48 47.3 0.13 0.34 1.68 54 30 300041 PRO A, 300175 LEU A, 300180 TYR A 4.38 48.04 0.3 0.72 1.11 54 31 300041 PRO A, 300175 LEU A, 300180 TYR A, 300173 ALA A 4.21 48.9 0.13 0.34 1.68 54 32 300041 PRO A, 300180 TYR A, 300173 ALA A 3.72 50.72 -1.1 0.07 2.19 54 33 300041 PRO A, 300180 TYR A, 300173 ALA A, 300153 GLU A 3.4 52.67 -1.7 -0.23 14.12 61 34 300041 PRO A, 300173 ALA A, 300153 GLU A, 300172 PRO A 3.43 54.99 -1.78 -0.53 14.11 64 35 300041 PRO A, 300153 GLU A, 300172 PRO A, 300041 ASN B 3.38 55.59 -2.55 -0.52 14.11 74 36 300041 PRO A, 300153 GLU A, 300172 PRO A, 300041 ASN B 3.32 56.21 -2.55 -0.52 14.11 74 37 300153 GLU A, 300172 PRO A, 300041 ASN B 3.06 57.08 -2.87 -0.67 18.29 79 38 300173 ALA A, 300153 GLU A, 300172 PRO A, 300041 ASN B 2.3 60.19 -2.25 -0.7 14.56 79 39 300173 ALA A, 300153 GLU A, 300041 ASN B, 300172 PRO A 2.43 60.72 -1.95 -0.88 15.01 90 40 300173 ALA A, 300153 GLU A, 300041 ASN B, 300172 PRO A, 300182 LEU A 2.57 61.1 -0.8 -0.47 12.03 78 41 300153 GLU A, 300041 ASN B, 300172 PRO A, 300182 LEU A, 300171 PHE A 2.7 61.41 -0.8 -0.47 12.03 78 42 300041 ASN B, 300172 PRO A, 300182 LEU A, 300171 PHE A 2.67 62.33 -0.13 -0.31 2.57 79 43 300041 ASN B, 300182 LEU A, 300171 PHE A 2.76 62.53 -0.03 -0.14 2.3 79 44 300041 ASN B, 300182 LEU A, 300170 THR A 2.76 62.82 -0.13 -0.13 1.72 88 45 300041 ASN B, 300182 LEU A, 300170 THR A, 300155 VAL A 2.71 64.03 -0.2 -0.3 2.14 88 46 300041 ASN B, 300170 THR A, 300155 VAL A 2.79 67.89 -1.53 -0.78 2.81 105 47 300041 ASN B, 300170 THR A, 300155 VAL A, 300156 THR A, 300157 VAL A 2.99 68.17 -1.14 -0.78 2.69 106 48 300041 ASN B, 300156 THR A, 300157 VAL A 3.06 68.47 -1.53 -0.78 2.81 105 49 300041 ASN B, 300170 THR A, 300156 THR A, 300157 VAL A 3.18 68.64 -1.33 -0.78 2.52 106 50 300041 ASN B, 300170 THR A, 300157 VAL A 3.19 68.75 -1.53 -0.78 2.81 105 51 300041 ASN B, 300170 THR A, 300155 VAL A 3.18 68.91 -1.53 -0.78 2.81 105 52 300041 ASN B, 300170 THR A, 300157 VAL A 3.23 69.12 -1.53 -0.78 2.81 105 53 300041 ASN B, 300170 THR A, 300156 THR A, 300157 VAL A 3.26 69.53 -1.33 -0.78 2.52 106 54 300041 ASN B, 300170 THR A, 300157 VAL A 3.26 72.79 -1.53 -0.78 2.81 105 55 300041 ASN B, 300170 THR A, 300157 VAL A, 300040 THR B 4.21 73.51 -1.25 -0.79 2.95 105 56 300041 ASN B, 300170 THR A, 300157 VAL A, 300040 THR B, 300168 VAL A 4.57 73.64 -1.08 -0.79 3.04 105 57 300170 THR A, 300157 VAL A, 300040 THR B, 300168 VAL A 4.62 73.78 0.68 -0.31 2.14 102 58 300170 THR A, 300157 VAL A, 300040 THR B, 300168 VAL A, 300169 LYS B 4.53 73.94 -1.16 -0.72 12.26 89 59 300157 VAL A, 300040 THR B, 300168 VAL A, 300169 LYS B 4.52 73.99 -0.13 -0.22 14.1 85 60 300170 THR A, 300157 VAL A, 300168 VAL A, 300169 LYS B 4.39 74.75 -0.2 -0.21 13.67 92 61 300157 VAL A, 300168 VAL A, 300169 LYS B, 300165 SER A 4.26 75.12 -0.13 -0.22 14.1 85 62 300168 VAL A, 300169 LYS B, 300165 SER A 1.97 79.73 -0.03 -0.03 17.67 85 63 300168 VAL A, 300169 LYS B, 300165 SER A 1.32 81.39 -1.57 -0.67 18.75 72 64 300168 VAL A, 300169 LYS B, 300165 SER A, 300167 GLY A 1.52 82.59 -1.28 -0.7 14.91 72 65 300168 VAL A, 300169 LYS B, 300167 GLY A 1.66 83.77 -1.57 -0.67 18.75 72 66 300168 VAL A, 300169 LYS B, 300167 GLY A, 300169 HIS A 1.84 84.06 -1.98 -0.44 26.97 81 67 300168 VAL A, 300169 LYS B, 300167 GLY A, 300169 HIS A 2 84.16 -1.98 -0.44 26.97 81 68 300168 VAL A, 300169 LYS B, 300167 GLY A, 300169 HIS A 1.86 84.6 -1.98 -0.44 26.97 81 69 300169 LYS B, 300167 GLY A, 300169 HIS A, 300167 ASP B 1.71 85.15 -2.75 -0.5 38.55 83 70 300169 LYS B, 300167 GLY A, 300167 ASP B 1.59 86.4 -2.6 -0.75 34.19 79 71 300169 LYS B, 300167 GLY A 1.57 87.13 -2.15 -0.61 26.44 72 72 300169 LYS B, 300167 GLY A, 300166 SER A 1.58 88.69 -1.57 -0.67 18.75 72 73 300169 LYS B, 300167 GLY A, 300166 SER A 1.77 92.1 -1.7 -0.73 18.18 94 74 300169 LYS B, 300167 GLY A, 300166 SER A, 300170 ASP B 3.44 93.66 -2.15 -0.81 26.06 91 75 300167 GLY A, 300166 SER A, 300170 ASP B, 300169 LYS B 4.28 94.11 -1.28 -0.9 14.53 101 76 300167 GLY A, 300166 SER A, 300170 ASP B 4.45 95.25 -1.57 -0.94 18.25 101 77 300167 GLY A, 300166 SER A, 300170 ASP B, 300140 MET A 4.93 96.14 -0.7 -0.45 14.05 98 78 300167 GLY A, 300170 ASP B, 300140 MET A 4.22 97.72 -0.67 -0.28 18.17 89 79 300167 GLY A, 300170 ASP B, 300140 MET A, 300138 ASN B 3.89 98.92 -1.38 -0.4 14.47 94 80 300170 ASP B, 300140 MET A, 300138 ASN B 3.95 100.48 -1.7 -0.27 18.17 94 81 300140 MET A, 300138 ASN B, 300187 THR A 3.57 102.42 -0.77 -0.18 2.16 101 82 300140 MET A, 300138 ASN B, 300187 THR A, 300114 THR B 2.84 103.86 -0.75 -0.33 2.03 102 83 300140 MET A, 300187 THR A, 300114 THR B 2.63 106.15 0.17 -0.18 1.58 102 84 300140 MET A, 300114 THR B 2.77 106.87 0.6 0.12 1.55 100 85 300140 MET A, 300114 THR B, 300138 ASN A 2.91 107.43 -0.77 -0.18 2.16 101 86 300140 MET A, 300114 THR B, 300138 ASN A, 300139 SER A 2.92 108.72 -0.78 -0.38 2.04 105 87 300140 MET A, 300114 THR B, 300138 ASN A 2.61 111.28 -0.77 -0.18 2.16 101 88 300114 THR B, 300138 ASN A 3.16 114.63 -2.1 -0.77 2.52 105 89 300114 THR B, 300138 ASN A, 300113 PRO B 3.78 115.19 -1.53 -0.78 2.81 105 90 300114 THR B, 300138 ASN A, 300113 PRO B, 300207 LYS B 3.67 115.74 -2.13 -0.69 14.48 94 91 300114 THR B, 300138 ASN A, 300207 LYS B 3.24 117.55 -2.7 -0.65 18.18 94 92 300114 THR B, 300138 ASN A, 300207 LYS B, 300115 VAL B 3.05 118.55 -2.13 -0.69 14.48 94 93 300114 THR B, 300138 ASN A, 300207 LYS B, 300136 GLN A 2.93 119.37 -2.13 -0.69 14.48 94 94 300114 THR B, 300138 ASN A, 300207 LYS B, 300115 VAL B, 300136 GLN A 2.76 120.34 -1.78 -0.71 12.26 94 95 300114 THR B, 300207 LYS B, 300115 VAL B, 300136 GLN A 2.71 120.71 -1.35 -0.7 14.48 89 96 300114 THR B, 300138 ASN A, 300207 LYS B, 300115 VAL B, 300136 GLN A 2.67 121.29 -1.78 -0.71 12.26 94 97 300114 THR B, 300138 ASN A, 300115 VAL B, 300136 GLN A, 300116 SER B 2.65 121.64 -1.16 -0.82 2.69 109 98 300114 THR B, 300115 VAL B, 300136 GLN A, 300116 SER B, 300140 MET A 2.61 123.16 -0.54 -0.83 2.69 112 99 300207 LYS B, 300115 VAL B, 300136 GLN A, 300116 SER B 2.59 123.79 -1.38 -0.75 14.48 94 100 300115 VAL B, 300136 GLN A, 300116 SER B 2.49 124.9 -0.53 -0.86 2.81 117 101 300115 VAL B, 300136 GLN A, 300116 SER B, 300135 ALA A 2.28 125.6 0.05 -0.64 2.11 108 102 300115 VAL B, 300116 SER B, 300135 ALA A 2.25 126.65 0.2 -0.58 1.68 108 103 300116 SER B, 300135 ALA A, 300117 ILE B 1.96 129.35 0.2 -0.58 1.68 108 104 300135 ALA A, 300117 ILE B, 300116 SER B 1.88 129.82 0.33 -0.53 2.25 100 105 300135 ALA A, 300116 SER B, 300117 ILE B 1.72 130.75 1.97 0.34 1.17 101 106 300135 ALA A, 300117 ILE B 1.67 131.82 3.15 0.92 0.07 101 107 300135 ALA A, 300117 ILE B, 300208 SER B 1.7 132.36 1.97 0.34 1.17 101 108 300135 ALA A, 300117 ILE B, 300208 SER B, 300132 GLY A 1.75 133.22 1.38 0.06 1.72 101 109 300135 ALA A, 300117 ILE B, 300132 GLY A 1.85 133.71 1.97 0.34 1.17 101 110 300117 ILE B, 300132 GLY A, 300209 PHE B 1.58 135.13 2.3 0.79 1.29 77 111 300117 ILE B, 300132 GLY A, 300209 PHE B, 300119 PRO B 1.56 135.57 0.1 -0.09 2.17 54 112 300117 ILE B, 300132 GLY A, 300119 PRO B 1.56 135.71 -0.8 -0.56 2.78 58 113 300117 ILE B, 300132 GLY A, 300119 PRO B 1.49 135.89 0.83 0.31 1.7 80 114 300117 ILE B, 300132 GLY A, 300119 PRO B 1.46 136.27 -0.8 -0.56 2.78 58 115 300132 GLY A, 300209 PHE B, 300119 PRO B 1.45 140.29 0.27 0.15 1.77 54 116 300132 GLY A, 300209 PHE B, 300119 PRO B, 300210 ASN B 1.96 140.78 0.1 -0.09 2.17 54 117 300132 GLY A, 300209 PHE B, 300119 PRO B, 300210 ASN B, 300133 SER A 2.08 141.35 0 -0.23 2.41 54 118 300132 GLY A, 300119 PRO B, 300210 ASN B, 300133 SER A, 300218 ARG A 2.22 142.36 -1.46 -0.58 12.74 70 119 300132 GLY A, 300119 PRO B, 300210 ASN B, 300218 ARG A 2.34 142.61 -1.73 -0.53 15.09 70 120 300119 PRO B, 300210 ASN B, 300218 ARG A 2.06 144.02 -2.17 -0.44 18.99 70 121 300209 PHE B, 300119 PRO B, 300210 ASN B, 300218 ARG A 2.05 144.49 -0.93 0.01 14.33 64 122 300119 PRO B, 300210 ASN B, 300218 ARG A, 300211 ARG B 1.99 145.18 -1.73 -0.53 15.09 70 123 300119 PRO B, 300210 ASN B, 300218 ARG A, 300211 ARG B, 300186 TYR B 1.96 145.33 -1.64 -0.2 12.39 63 124 300119 PRO B, 300218 ARG A, 300211 ARG B, 300186 TYR B 1.93 146.63 -1.95 -0.05 14.64 63 125 300218 ARG A, 300211 ARG B, 300186 TYR B 2.04 147.3 -2.07 -0.04 19 66 126 300218 ARG A, 300186 TYR B, 300211 ARG B 2.15 148.79 -3.43 0.09 35.2 72 127 300218 ARG A, 300186 TYR B, 300211 ARG B, 300125 LEU B 2.41 148.79 -1.63 0.35 26.44 67 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
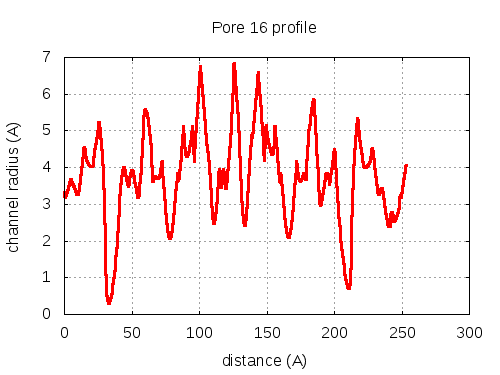
Unique lining residues set - all
46 GLU A, 61 ASN A, 63 LYS A, 3 LEU B, 98 PHE B, 64 ILE A, 200020 SER B, 63 LYS A, 200018 ARG B, 40 ARG A, 88 SER A, 91 SER A, 91 SER A, 41 PRO A, 88 SER A, 175 LEU A, 180 TYR A, 173 ALA A, 153 GLU A, 172 PRO A, 41 ASN B, 172 PRO A, 182 LEU A, 171 PHE A, 170 THR A, 155 VAL A, 156 THR A, 157 VAL A, 40 THR B, 168 VAL A, 168 VAL A, 169 LYS B, 165 SER A, 167 GLY A, 169 HIS A, 167 ASP B, 166 SER A, 166 SER A, 170 ASP B, 169 LYS B, 140 MET A, 138 ASN B, 187 THR A, 114 THR B, 138 ASN A, 139 SER A, 113 PRO B, 207 LYS B, 115 VAL B, 136 GLN A, 116 SER B, 140 MET A, 135 ALA A, 117 ILE B, 116 SER B, 117 ILE B, 208 SER B, 132 GLY A, 209 PHE B, 119 PRO B, 210 ASN B, 133 SER A, 218 ARG A, 211 ARG B, 186 TYR B, 211 ARG B, 125 LEU B
Unique lining residues set - sidechains
46 GLU A, 61 ASN A, 63 LYS A, 3 LEU B, 64 ILE A, 200020 SER B, 200018 ARG B, 40 ARG A, 91 SER A, 41 PRO A, 88 SER A, 175 LEU A, 180 TYR A, 153 GLU A, 172 PRO A, 41 ASN B, 182 LEU A, 170 THR A, 156 THR A, 168 VAL A, 169 LYS B, 169 HIS A, 167 ASP B, 166 SER A, 170 ASP B, 140 MET A, 138 ASN B, 187 THR A, 114 THR B, 138 ASN A, 139 SER A, 207 LYS B, 116 SER B, 135 ALA A, 117 ILE B, 209 PHE B, 119 PRO B, 218 ARG A, 186 TYR B, 211 ARG B, 125 LEU B
Physicochemical properties of lining side-chains
Charge: 3 (7-4)
Hydropathy: -1
Hydrophobicity: -0.37
Polarity: 11.83
Mutability: 86
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 46 GLU A, 61 ASN A, 63 LYS A, 3 LEU B, 98 PHE B 4.26 1.42 -1.5 -0.4 21.26 76 2 46 GLU A, 63 LYS A, 3 LEU B, 98 PHE B 4.73 2.74 -1 -0.3 25.73 67 3 46 GLU A, 63 LYS A, 3 LEU B, 64 ILE A 5.15 5.23 0.23 0.35 24.92 76 4 46 GLU A, 63 LYS A, 3 LEU B, 200020 SER B 5.38 6.05 -1.1 -0.35 25.3 80 5 46 GLU A, 63 LYS A, 64 ILE A 2.92 10.86 -0.97 0.09 33.18 84 6 46 GLU A, 64 ILE A, 63 LYS A 3.05 11.69 0.2 -0.04 17.8 90 7 46 GLU A, 64 ILE A, 63 LYS A, 200018 ARG B 3.31 15.01 -0.98 -0.14 26.35 87 8 46 GLU A, 64 ILE A, 200018 ARG B, 40 ARG A 3.53 18 -2 -0.04 38.51 86 9 46 GLU A, 200018 ARG B, 40 ARG A 3.92 20.52 -4.17 -0.66 51.3 81 10 46 GLU A, 64 ILE A, 40 ARG A 4.05 21.59 -1.17 0.08 34.01 87 11 46 GLU A, 40 ARG A 2.98 24.09 -4 -0.78 50.95 80 12 40 ARG A 1.23 30.7 -4.5 -0.42 52 83 13 40 ARG A, 88 SER A 1.25 31.59 -2.45 -0.61 27.69 83 14 40 ARG A, 88 SER A, 91 SER A 1.51 32.05 -1.77 -0.67 19.59 83 15 40 ARG A, 88 SER A, 91 SER A 1.91 32.31 -1.9 -0.73 19.02 100 16 40 ARG A, 88 SER A, 91 SER A, 41 PRO A 2.35 32.59 -1.83 -0.57 14.66 86 17 40 ARG A, 88 SER A, 91 SER A, 41 PRO A 2.74 33.41 -1.73 -0.53 15.09 70 18 40 ARG A, 88 SER A, 41 PRO A 3.23 36.07 -2.17 -0.44 18.99 70 19 40 ARG A, 41 PRO A, 88 SER A 4.55 37.86 -2.3 -0.49 18.42 86 20 41 PRO A, 88 SER A 4.79 39.33 -1.2 -0.53 1.63 87 21 91 SER A, 41 PRO A, 88 SER A 4.69 39.81 -1.07 -0.68 1.64 97 22 91 SER A, 41 PRO A, 88 SER A, 175 LEU A 4.6 40.23 0.15 -0.22 1.26 86 23 41 PRO A, 88 SER A, 175 LEU A, 180 TYR A 4.5 40.53 0.02 0.3 1.25 69 24 91 SER A, 88 SER A, 175 LEU A, 180 TYR A 4.27 40.95 0.23 0.08 1.27 84 25 91 SER A, 88 SER A, 180 TYR A 4.02 41.52 -0.97 -0.28 1.65 94 26 91 SER A, 88 SER A, 175 LEU A, 180 TYR A 4.01 42.17 0.23 0.08 1.27 84 27 88 SER A, 91 SER A, 41 PRO A, 175 LEU A, 180 TYR A 4.02 43.08 -0.06 0.08 1.67 69 28 41 PRO A, 175 LEU A, 180 TYR A 4.07 46.98 0.3 0.72 1.11 54 29 41 PRO A, 175 LEU A, 180 TYR A, 173 ALA A 4.48 47.3 0.13 0.34 1.68 54 30 41 PRO A, 175 LEU A, 180 TYR A 4.38 48.04 0.3 0.72 1.11 54 31 41 PRO A, 175 LEU A, 180 TYR A, 173 ALA A 4.21 48.9 0.13 0.34 1.68 54 32 41 PRO A, 180 TYR A, 173 ALA A 3.72 50.72 -1.1 0.07 2.19 54 33 41 PRO A, 180 TYR A, 173 ALA A, 153 GLU A 3.4 52.67 -1.7 -0.23 14.12 61 34 41 PRO A, 173 ALA A, 153 GLU A, 172 PRO A 3.43 54.99 -1.78 -0.53 14.11 64 35 41 PRO A, 153 GLU A, 172 PRO A, 41 ASN B 3.38 55.59 -2.55 -0.52 14.11 74 36 41 PRO A, 153 GLU A, 172 PRO A, 41 ASN B 3.32 56.21 -2.55 -0.52 14.11 74 37 153 GLU A, 172 PRO A, 41 ASN B 3.06 57.08 -2.87 -0.67 18.29 79 38 173 ALA A, 153 GLU A, 172 PRO A, 41 ASN B 2.3 60.19 -2.25 -0.7 14.56 79 39 173 ALA A, 153 GLU A, 41 ASN B, 172 PRO A 2.43 60.72 -1.95 -0.88 15.01 90 40 173 ALA A, 153 GLU A, 41 ASN B, 172 PRO A, 182 LEU A 2.57 61.1 -0.8 -0.47 12.03 78 41 153 GLU A, 41 ASN B, 172 PRO A, 182 LEU A, 171 PHE A 2.7 61.41 -0.8 -0.47 12.03 78 42 41 ASN B, 172 PRO A, 182 LEU A, 171 PHE A 2.67 62.33 -0.13 -0.31 2.57 79 43 41 ASN B, 182 LEU A, 171 PHE A 2.76 62.53 -0.03 -0.14 2.3 79 44 41 ASN B, 182 LEU A, 170 THR A 2.76 62.82 -0.13 -0.13 1.72 88 45 41 ASN B, 182 LEU A, 170 THR A, 155 VAL A 2.71 64.03 -0.2 -0.3 2.14 88 46 41 ASN B, 170 THR A, 155 VAL A 2.79 67.89 -1.53 -0.78 2.81 105 47 41 ASN B, 170 THR A, 155 VAL A, 156 THR A, 157 VAL A 2.99 68.17 -1.14 -0.78 2.69 106 48 41 ASN B, 156 THR A, 157 VAL A 3.06 68.48 -1.53 -0.78 2.81 105 49 41 ASN B, 170 THR A, 156 THR A, 157 VAL A 3.19 68.64 -1.33 -0.78 2.52 106 50 41 ASN B, 170 THR A, 157 VAL A 3.19 68.75 -1.53 -0.78 2.81 105 51 41 ASN B, 170 THR A, 155 VAL A 3.18 68.9 -1.53 -0.78 2.81 105 52 41 ASN B, 170 THR A, 157 VAL A 3.23 69.12 -1.53 -0.78 2.81 105 53 41 ASN B, 170 THR A, 156 THR A, 157 VAL A 3.26 69.53 -1.33 -0.78 2.52 106 54 41 ASN B, 170 THR A, 157 VAL A 3.26 72.79 -1.53 -0.78 2.81 105 55 41 ASN B, 170 THR A, 157 VAL A, 40 THR B 4.21 73.51 -1.25 -0.79 2.95 105 56 41 ASN B, 170 THR A, 157 VAL A, 40 THR B, 168 VAL A 4.57 73.64 -1.08 -0.79 3.04 105 57 170 THR A, 157 VAL A, 40 THR B, 168 VAL A 4.62 73.78 0.68 -0.31 2.14 102 58 170 THR A, 157 VAL A, 40 THR B, 168 VAL A, 169 LYS B 4.53 73.94 -1.16 -0.72 12.26 89 59 157 VAL A, 40 THR B, 168 VAL A, 169 LYS B 4.52 73.99 -0.13 -0.22 14.1 85 60 170 THR A, 157 VAL A, 168 VAL A, 169 LYS B 4.39 74.75 -0.2 -0.21 13.67 92 61 157 VAL A, 168 VAL A, 169 LYS B, 165 SER A 4.26 75.12 -0.13 -0.22 14.1 85 62 168 VAL A, 169 LYS B, 165 SER A 1.97 79.73 -0.03 -0.03 17.67 85 63 168 VAL A, 169 LYS B, 165 SER A 1.32 81.39 -1.57 -0.67 18.75 72 64 168 VAL A, 169 LYS B, 165 SER A, 167 GLY A 1.52 82.59 -1.28 -0.7 14.91 72 65 168 VAL A, 169 LYS B, 167 GLY A 1.66 83.77 -1.57 -0.67 18.75 72 66 168 VAL A, 169 LYS B, 167 GLY A, 169 HIS A 1.84 84.06 -1.98 -0.44 26.97 81 67 168 VAL A, 169 LYS B, 167 GLY A, 169 HIS A 2 84.16 -1.98 -0.44 26.97 81 68 168 VAL A, 169 LYS B, 167 GLY A, 169 HIS A 1.86 84.6 -1.98 -0.44 26.97 81 69 169 LYS B, 167 GLY A, 169 HIS A, 167 ASP B 1.71 85.15 -2.75 -0.5 38.55 83 70 169 LYS B, 167 GLY A, 167 ASP B 1.59 86.4 -2.6 -0.75 34.19 79 71 169 LYS B, 167 GLY A 1.57 87.13 -2.15 -0.61 26.44 72 72 169 LYS B, 167 GLY A, 166 SER A 1.58 88.69 -1.57 -0.67 18.75 72 73 169 LYS B, 167 GLY A, 166 SER A 1.77 92.09 -1.7 -0.73 18.18 94 74 169 LYS B, 167 GLY A, 166 SER A, 170 ASP B 3.44 93.66 -2.15 -0.81 26.06 91 75 167 GLY A, 166 SER A, 170 ASP B, 169 LYS B 4.28 94.11 -1.28 -0.9 14.53 101 76 167 GLY A, 166 SER A, 170 ASP B 4.45 95.25 -1.57 -0.94 18.25 101 77 167 GLY A, 166 SER A, 170 ASP B, 140 MET A 4.93 96.14 -0.7 -0.45 14.05 98 78 167 GLY A, 170 ASP B, 140 MET A 4.22 97.72 -0.67 -0.28 18.17 89 79 167 GLY A, 170 ASP B, 140 MET A, 138 ASN B 3.89 98.92 -1.38 -0.4 14.47 94 80 170 ASP B, 140 MET A, 138 ASN B 3.95 100.48 -1.7 -0.27 18.17 94 81 140 MET A, 138 ASN B, 187 THR A 3.57 102.42 -0.77 -0.18 2.16 101 82 140 MET A, 138 ASN B, 187 THR A, 114 THR B 2.84 103.86 -0.75 -0.33 2.03 102 83 140 MET A, 187 THR A, 114 THR B 2.63 106.15 0.17 -0.18 1.58 102 84 140 MET A, 114 THR B 2.77 106.87 0.6 0.12 1.55 100 85 140 MET A, 114 THR B, 138 ASN A 2.91 107.43 -0.77 -0.18 2.16 101 86 140 MET A, 114 THR B, 138 ASN A, 139 SER A 2.92 108.71 -0.78 -0.38 2.04 105 87 140 MET A, 114 THR B, 138 ASN A 2.61 111.28 -0.77 -0.18 2.16 101 88 114 THR B, 138 ASN A 3.16 114.63 -2.1 -0.77 2.52 105 89 114 THR B, 138 ASN A, 113 PRO B 3.78 115.19 -1.53 -0.78 2.81 105 90 114 THR B, 138 ASN A, 113 PRO B, 207 LYS B 3.67 115.74 -2.13 -0.69 14.48 94 91 114 THR B, 138 ASN A, 207 LYS B 3.24 117.55 -2.7 -0.65 18.18 94 92 114 THR B, 138 ASN A, 207 LYS B, 115 VAL B 3.05 118.54 -2.13 -0.69 14.48 94 93 114 THR B, 138 ASN A, 207 LYS B, 136 GLN A 2.93 119.36 -2.13 -0.69 14.48 94 94 114 THR B, 138 ASN A, 207 LYS B, 115 VAL B, 136 GLN A 2.76 120.34 -1.78 -0.71 12.26 94 95 114 THR B, 207 LYS B, 115 VAL B, 136 GLN A 2.71 120.71 -1.35 -0.7 14.48 89 96 114 THR B, 138 ASN A, 207 LYS B, 115 VAL B, 136 GLN A 2.67 121.29 -1.78 -0.71 12.26 94 97 114 THR B, 138 ASN A, 115 VAL B, 136 GLN A, 116 SER B 2.65 121.64 -1.16 -0.82 2.69 109 98 114 THR B, 115 VAL B, 136 GLN A, 116 SER B, 140 MET A 2.61 123.15 -0.54 -0.83 2.69 112 99 207 LYS B, 115 VAL B, 136 GLN A, 116 SER B 2.59 123.79 -1.38 -0.75 14.48 94 100 115 VAL B, 136 GLN A, 116 SER B 2.49 124.9 -0.53 -0.86 2.81 117 101 115 VAL B, 136 GLN A, 116 SER B, 135 ALA A 2.28 125.6 0.05 -0.64 2.11 108 102 115 VAL B, 116 SER B, 135 ALA A 2.25 126.65 0.2 -0.58 1.68 108 103 116 SER B, 135 ALA A, 117 ILE B 1.96 129.35 0.2 -0.58 1.68 108 104 135 ALA A, 117 ILE B, 116 SER B 1.88 129.81 0.33 -0.53 2.25 100 105 135 ALA A, 116 SER B, 117 ILE B 1.72 130.75 1.97 0.34 1.17 101 106 135 ALA A, 117 ILE B 1.67 131.82 3.15 0.92 0.07 101 107 135 ALA A, 117 ILE B, 208 SER B 1.7 132.36 1.97 0.34 1.17 101 108 135 ALA A, 117 ILE B, 208 SER B, 132 GLY A 1.75 133.22 1.38 0.06 1.72 101 109 135 ALA A, 117 ILE B, 132 GLY A 1.85 133.71 1.97 0.34 1.17 101 110 117 ILE B, 132 GLY A, 209 PHE B 1.58 135.13 2.3 0.79 1.29 77 111 117 ILE B, 132 GLY A, 209 PHE B, 119 PRO B 1.56 135.57 0.1 -0.09 2.17 54 112 117 ILE B, 132 GLY A, 119 PRO B 1.56 135.7 -0.8 -0.56 2.78 58 113 117 ILE B, 132 GLY A, 119 PRO B 1.49 135.89 0.83 0.31 1.7 80 114 117 ILE B, 132 GLY A, 119 PRO B 1.46 136.27 -0.8 -0.56 2.78 58 115 132 GLY A, 209 PHE B, 119 PRO B 1.45 140.29 0.27 0.15 1.77 54 116 132 GLY A, 209 PHE B, 119 PRO B, 210 ASN B 1.96 140.78 0.1 -0.09 2.17 54 117 132 GLY A, 209 PHE B, 119 PRO B, 210 ASN B, 133 SER A 2.08 141.35 0 -0.23 2.41 54 118 132 GLY A, 119 PRO B, 210 ASN B, 133 SER A, 218 ARG A 2.22 142.36 -1.46 -0.58 12.74 70 119 132 GLY A, 119 PRO B, 210 ASN B, 218 ARG A 2.34 142.61 -1.73 -0.53 15.09 70 120 119 PRO B, 210 ASN B, 218 ARG A 2.05 144.02 -2.17 -0.44 18.99 70 121 209 PHE B, 119 PRO B, 210 ASN B, 218 ARG A 2.05 144.49 -0.93 0.01 14.33 64 122 119 PRO B, 210 ASN B, 218 ARG A, 211 ARG B 1.99 145.18 -1.73 -0.53 15.09 70 123 119 PRO B, 210 ASN B, 218 ARG A, 211 ARG B, 186 TYR B 1.96 145.33 -1.64 -0.2 12.39 63 124 119 PRO B, 218 ARG A, 211 ARG B, 186 TYR B 1.93 146.63 -1.95 -0.05 14.64 63 125 218 ARG A, 211 ARG B, 186 TYR B 2.04 147.3 -2.07 -0.04 19 66 126 218 ARG A, 186 TYR B, 211 ARG B 2.15 148.79 -3.43 0.09 35.2 72 127 218 ARG A, 186 TYR B, 211 ARG B, 125 LEU B 2.41 148.79 -1.63 0.35 26.44 67 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
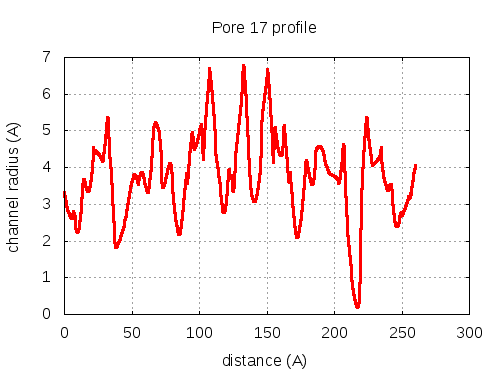
Unique lining residues set - all
200042 GLY A, 200040 THR B, 200085 ASN B, 200165 ASP B, 200041 ASN B, 200172 PRO A, 200041 PRO A, 200153 GLU A, 200173 ALA A, 200180 TYR A, 200175 LEU A, 200088 SER A, 200091 SER A, 200088 SER A, 200040 ARG A, 200091 SER A, 200043 HIS A, 200046 GLU A, 100018 ARG B, 200064 ILE A, 200063 LYS A, 200063 LYS A, 100020 SER B, 200003 LEU B, 200098 PHE B, 200061 ASN A, 200097 THR B, 200001 ASP B, 100072 THR B, 100070 ASP B, 200001 ASP B, 200026 SER B, 100024 ARG B, 200026 SER B, 100069 THR B, 100026 SER B, 100027 GLN B, 100028 SER B, 100028 SER B, 200027 GLN B, 100027 GLN B, 200028 SER B, 200028 SER B, 27 GLN B, 28 SER B, 300027 GLN B, 300028 SER B, 200093 ARG B, 200082 TYR C, 100093 ARG B, 100079 GLY C, 200079 GLY C, 300093 ARG B, 79 GLY C, 100082 TYR C, 300079 GLY C, 300082 TYR C, 93 ARG B, 82 TYR C, 200058 GLN C, 200080 ASP C, 200030 GLY B, 200092 ASN B, 84 VAL C, 200064 ARG C, 200032 ASP B, 200031 THR B, 200031 THR B, 84 VAL C, 200050 TYR B, 200053 GLU B, 85 THR C, 85 THR C, 54 ALA C, 53 GLY C, 57 ARG A
Unique lining residues set - sidechains
200040 THR B, 200085 ASN B, 200165 ASP B, 200041 ASN B, 200172 PRO A, 200041 PRO A, 200153 GLU A, 200180 TYR A, 200175 LEU A, 200091 SER A, 200088 SER A, 200040 ARG A, 200043 HIS A, 200046 GLU A, 100018 ARG B, 200064 ILE A, 200063 LYS A, 100020 SER B, 200003 LEU B, 200061 ASN A, 200097 THR B, 200001 ASP B, 100072 THR B, 100070 ASP B, 200026 SER B, 100024 ARG B, 100069 THR B, 100028 SER B, 200027 GLN B, 100027 GLN B, 200028 SER B, 27 GLN B, 28 SER B, 300027 GLN B, 200093 ARG B, 200082 TYR C, 100093 ARG B, 300093 ARG B, 100082 TYR C, 300082 TYR C, 93 ARG B, 82 TYR C, 200058 GLN C, 200092 ASN B, 84 VAL C, 200064 ARG C, 200032 ASP B, 200031 THR B, 200050 TYR B, 200053 GLU B, 85 THR C, 54 ALA C, 57 ARG A
Physicochemical properties of lining side-chains
Charge: 3 (10-7)
Hydropathy: -1.6
Hydrophobicity: -0.4
Polarity: 16.06
Mutability: 86
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 200042 GLY A, 200040 THR B, 200085 ASN B, 200165 ASP B 3.31 0.14 -2.03 -0.85 14.53 99 2 200042 GLY A, 200040 THR B, 200165 ASP B 3.13 1.96 -1.53 -0.87 18.25 96 3 200042 GLY A, 200040 THR B, 200165 ASP B, 200041 ASN B 3.19 4.02 -2.03 -0.85 14.53 99 4 200042 GLY A, 200040 THR B, 200165 ASP B, 200041 ASN B, 200172 PRO A 3.69 4.22 -1.94 -0.69 11.94 88 5 200042 GLY A, 200165 ASP B, 200041 ASN B, 200172 PRO A 3.73 4.79 -2.25 -0.68 14.51 82 6 200042 GLY A, 200041 ASN B, 200172 PRO A, 200041 PRO A 3.71 4.95 -1.78 -0.44 2.48 73 7 200042 GLY A, 200041 ASN B, 200172 PRO A, 200041 PRO A 3.64 5.45 -1.78 -0.44 2.48 73 8 200042 GLY A, 200041 ASN B, 200172 PRO A, 200041 PRO A 3.55 6.46 -1.78 -0.44 2.48 73 9 200041 ASN B, 200172 PRO A, 200041 PRO A, 200153 GLU A 3.52 7.01 -2.55 -0.52 14.11 74 10 200041 ASN B, 200172 PRO A, 200041 PRO A, 200153 GLU A 3.49 7.85 -2.55 -0.52 14.11 74 11 200172 PRO A, 200041 PRO A, 200153 GLU A, 200173 ALA A 3.36 9.93 -1.78 -0.53 14.11 64 12 200041 PRO A, 200153 GLU A, 200173 ALA A, 200180 TYR A 3.31 12.2 -1.7 -0.23 14.12 61 13 200041 PRO A, 200173 ALA A, 200180 TYR A 3.48 14.18 -1.1 0.07 2.19 54 14 200041 PRO A, 200173 ALA A, 200180 TYR A, 200175 LEU A 4.38 14.69 0.13 0.34 1.68 54 15 200041 PRO A, 200180 TYR A, 200175 LEU A 4.37 15.36 0.3 0.72 1.11 54 16 200041 PRO A, 200173 ALA A, 200180 TYR A, 200175 LEU A 4.44 15.72 0.13 0.34 1.68 54 17 200041 PRO A, 200180 TYR A, 200175 LEU A 4.33 20.33 0.3 0.72 1.11 54 18 200041 PRO A, 200180 TYR A, 200175 LEU A, 200088 SER A, 200091 SER A 4.29 20.95 -0.06 0.08 1.67 69 19 200041 PRO A, 200180 TYR A, 200175 LEU A, 200088 SER A 4.23 21.32 0.02 0.3 1.25 69 20 200180 TYR A, 200091 SER A, 200088 SER A 4.18 21.47 -0.97 -0.28 1.65 94 21 200091 SER A, 200088 SER A 4.14 21.54 -0.8 -0.97 1.67 117 22 200180 TYR A, 200091 SER A, 200088 SER A 4.12 21.86 -0.97 -0.28 1.65 94 23 200180 TYR A, 200175 LEU A, 200091 SER A, 200088 SER A 4.23 22.08 0.23 0.08 1.27 84 24 200041 PRO A, 200180 TYR A, 200175 LEU A, 200088 SER A 4.33 22.76 0.02 0.3 1.25 69 25 200041 PRO A, 200175 LEU A, 200091 SER A, 200088 SER A 4.56 23.19 0.15 -0.22 1.26 86 26 200041 PRO A, 200091 SER A, 200088 SER A 4.66 24.12 -1.07 -0.68 1.64 97 27 200041 PRO A, 200088 SER A 4.88 24.9 -1.2 -0.53 1.63 87 28 200041 PRO A, 200088 SER A, 200040 ARG A 4.22 27.7 -2.3 -0.49 18.42 86 29 200041 PRO A, 200088 SER A, 200040 ARG A 2.96 29.76 -2.17 -0.44 18.99 70 30 200041 PRO A, 200088 SER A, 200040 ARG A, 200091 SER A 2.78 30.09 -1.73 -0.53 15.09 70 31 200088 SER A, 200040 ARG A, 200091 SER A 2.27 31.61 -1.77 -0.67 19.59 83 32 200088 SER A, 200040 ARG A 1.97 32.63 -2.45 -0.61 27.69 83 33 200040 ARG A 0.8 38.25 -4.5 -0.42 52 83 34 200040 ARG A, 200043 HIS A 1.11 39.41 -3.85 -0.08 51.8 87 35 200040 ARG A, 200046 GLU A 1.7 43.5 -4 -0.78 50.95 80 36 200040 ARG A, 200046 GLU A, 100018 ARG B 4.03 46.08 -4.17 -0.66 51.3 81 37 200040 ARG A, 200046 GLU A, 100018 ARG B, 200064 ILE A 3.53 49.67 -2 -0.04 38.51 86 38 200046 GLU A, 100018 ARG B, 200064 ILE A, 200063 LYS A 3.66 52.85 -0.98 -0.14 26.35 87 39 200046 GLU A, 200064 ILE A, 200063 LYS A 3.42 53.61 0.2 -0.04 17.8 90 40 200046 GLU A, 200064 ILE A, 200063 LYS A 2.96 57.97 -0.97 0.09 33.18 84 41 200046 GLU A, 200064 ILE A, 200063 LYS A, 100020 SER B 5.25 58.49 -0.93 -0.18 25.3 92 42 200046 GLU A, 200063 LYS A, 100020 SER B, 200003 LEU B 5.42 59.26 -1.1 -0.35 25.3 80 43 200046 GLU A, 200064 ILE A, 200063 LYS A, 200003 LEU B 4.65 63.26 0.23 0.35 24.92 76 44 200046 GLU A, 200063 LYS A, 200003 LEU B, 200098 PHE B 4.45 64.07 -1 -0.3 25.73 67 45 200046 GLU A, 200063 LYS A, 200003 LEU B, 200098 PHE B, 200061 ASN A 4.3 64.54 -1.5 -0.4 21.26 76 46 200046 GLU A, 200063 LYS A, 200098 PHE B, 200061 ASN A, 200097 THR B 4.21 64.74 -2.4 -0.78 21.56 90 47 200046 GLU A, 200098 PHE B, 200061 ASN A, 200097 THR B 4.17 64.9 -2.03 -0.87 14.58 96 48 200046 GLU A, 200063 LYS A, 200098 PHE B, 200061 ASN A, 200097 THR B 4.17 65.1 -2.4 -0.78 21.56 90 49 200063 LYS A, 200003 LEU B, 200098 PHE B, 200061 ASN A, 200097 THR B 3.95 65.91 -0.94 -0.32 11.61 84 50 200063 LYS A, 200003 LEU B, 200098 PHE B, 200097 THR B 3.82 66.3 -0.3 -0.21 13.67 77 51 200063 LYS A, 200003 LEU B, 200061 ASN A, 200097 THR B 3.7 66.71 -1.08 -0.2 13.67 84 52 200063 LYS A, 200003 LEU B, 200097 THR B, 200001 ASP B 3.47 68.62 -1.08 -0.27 25.25 79 53 200063 LYS A, 200003 LEU B, 200001 ASP B 3.56 70.32 -1.2 -0.1 33.11 70 54 200063 LYS A, 200003 LEU B, 200001 ASP B, 100072 THR B 4.02 71.25 -1.08 -0.27 25.25 79 55 200063 LYS A, 200003 LEU B, 200001 ASP B, 100072 THR B, 100070 ASP B 4.2 71.45 -1.56 -0.42 30.14 81 56 200003 LEU B, 200001 ASP B, 100072 THR B, 100070 ASP B 3.96 72.44 -0.98 -0.43 25.3 83 57 200003 LEU B, 100070 ASP B, 200001 ASP B 2.95 74.86 -0.03 -0.23 17.74 70 58 200003 LEU B, 100070 ASP B 2.22 77.39 0.15 0.05 24.92 70 59 200003 LEU B, 100070 ASP B, 200026 SER B 2.13 80.22 -0.17 -0.29 17.17 85 60 200003 LEU B, 100070 ASP B, 200026 SER B, 100024 ARG B 2.51 81.89 -1.25 -0.32 25.88 85 61 100070 ASP B, 200026 SER B, 100024 ARG B 3.17 83.69 -2.93 -0.81 34.46 95 62 100070 ASP B, 100024 ARG B, 200026 SER B 3.64 84.68 -2.8 -0.75 35.03 84 63 100070 ASP B, 100024 ARG B, 200026 SER B, 100069 THR B 3.71 86.65 -2.28 -0.76 26.69 92 64 100024 ARG B, 200026 SER B, 100069 THR B 4.4 87.96 -1.87 -0.66 19.01 95 65 100024 ARG B, 200026 SER B, 100069 THR B, 100026 SER B 4.97 88.73 -1.5 -0.7 15.11 95 66 200026 SER B, 100069 THR B, 100026 SER B 4.4 91.59 -0.5 -0.79 2.81 107 67 200026 SER B, 100069 THR B, 100026 SER B, 100027 GLN B 4.39 92.49 -0.48 -0.79 2.95 107 68 200026 SER B, 100069 THR B, 100026 SER B, 100027 GLN B, 100028 SER B 4.48 94.57 -0.46 -0.79 3.04 107 69 200026 SER B, 100027 GLN B, 100028 SER B, 200027 GLN B 5.17 94.87 -1.28 -0.92 2.99 100 70 200026 SER B, 100069 THR B, 100028 SER B, 200027 GLN B 4.16 98.08 -1.35 -0.91 2.56 102 71 100027 GLN B, 100028 SER B, 200027 GLN B 5.34 98.73 -1.57 -0.96 2.86 100 72 100028 SER B, 200027 GLN B, 100027 GLN B 5.62 99.31 -2.6 -1.06 2.91 95 73 100028 SER B, 200027 GLN B, 100027 GLN B, 200028 SER B 5.85 99.78 -2.05 -0.99 3.03 95 74 100028 SER B, 200027 GLN B, 200028 SER B 6.05 100.08 -1.7 -1.01 2.29 106 75 27 GLN B, 28 SER B, 300027 GLN B, 300028 SER B 6.23 100.99 -2.05 -0.99 3.03 95 76 100028 SER B, 200027 GLN B, 200028 SER B 4.89 104.47 -1.7 -1.01 2.29 106 77 100028 SER B, 200027 GLN B, 200028 SER B, 200093 ARG B 4.78 104.81 -2.4 -0.87 14.72 100 78 200027 GLN B, 200028 SER B, 200093 ARG B 4.82 105.39 -2.93 -0.83 19.07 94 79 100028 SER B, 200027 GLN B, 200028 SER B, 200093 ARG B 4.75 105.84 -2.4 -0.87 14.72 100 80 100028 SER B, 200027 GLN B, 200028 SER B, 200093 ARG B 4.51 106.45 -2.3 -0.82 15.15 94 81 100028 SER B, 200027 GLN B, 200093 ARG B 2.47 111.62 -2.93 -0.83 19.07 94 82 100028 SER B, 200093 ARG B 3.06 112.3 -2.65 -0.7 26.84 100 83 100028 SER B, 200093 ARG B, 200082 TYR C 3.37 113.38 -2.2 -0.09 18.43 83 84 100028 SER B, 200093 ARG B, 200082 TYR C, 100093 ARG B 4.01 116.3 -2.78 -0.18 26.82 83 85 100028 SER B, 200093 ARG B, 200082 TYR C, 100093 ARG B, 100079 GLY C 5.99 117.1 -2.3 -0.3 22.13 83 86 200093 ARG B, 200082 TYR C, 100093 ARG B, 100079 GLY C, 200079 GLY C 6.52 118.03 -2.22 -0.27 22.47 72 87 300093 ARG B, 79 GLY C, 100082 TYR C, 300079 GLY C, 300082 TYR C 6.73 118.25 -1.58 0.04 12.4 61 88 300093 ARG B, 100082 TYR C, 300079 GLY C, 300082 TYR C, 93 ARG B 6.42 118.61 -2.4 0.12 22.12 66 89 100093 ARG B, 300093 ARG B, 100082 TYR C, 300079 GLY C, 300082 TYR C 6.3 119.03 -2.4 0.12 22.12 66 90 200093 ARG B, 200082 TYR C, 100093 ARG B, 100079 GLY C, 200079 GLY C 6.15 119.71 -2.22 -0.27 22.47 72 91 200093 ARG B, 200082 TYR C, 100079 GLY C, 200079 GLY C, 82 TYR C 5.57 121.53 -1.58 0.04 12.4 61 92 200093 ARG B, 200082 TYR C, 200079 GLY C, 82 TYR C 5.07 122.47 -1.88 0.25 14.65 61 93 200093 ARG B, 200082 TYR C, 200079 GLY C, 200058 GLN C 3.24 124.79 -2.43 -0.3 15.13 72 94 200093 ARG B, 200079 GLY C, 200058 GLN C, 200080 ASP C 3.37 125.49 -2.2 -0.78 15.57 83 95 200093 ARG B, 200079 GLY C, 82 TYR C, 200058 GLN C 3.75 126.58 -2.43 -0.3 15.13 72 96 200093 ARG B, 82 TYR C, 200058 GLN C, 200080 ASP C 3.67 126.78 -2.43 -0.3 15.13 72 97 200093 ARG B, 82 TYR C, 200058 GLN C 3.55 127.81 -3.1 -0.14 19.05 72 98 200028 SER B, 200093 ARG B, 82 TYR C, 200058 GLN C 3.63 128.03 -2.43 -0.3 15.13 72 99 200028 SER B, 200093 ARG B, 82 TYR C, 200058 GLN C 3.69 128.41 -2.53 -0.35 14.7 83 100 200028 SER B, 200093 ARG B, 82 TYR C 3.84 128.48 -2.2 -0.09 18.43 83 101 200028 SER B, 200093 ARG B, 82 TYR C, 200058 GLN C 3.67 129.25 -2.53 -0.35 14.7 83 102 200028 SER B, 82 TYR C, 200058 GLN C 3.23 131.52 -1.87 -0.32 2.27 83 103 200028 SER B, 82 TYR C, 200058 GLN C, 200030 GLY B 3.11 132.57 -1.5 -0.44 2.55 83 104 82 TYR C, 200058 GLN C, 200030 GLY B, 200092 ASN B 3.11 134.62 -2.18 -0.39 2.98 79 105 82 TYR C, 200030 GLY B, 200092 ASN B 3.18 134.94 -1.73 -0.15 2.79 77 106 82 TYR C, 200030 GLY B, 200092 ASN B, 84 VAL C 3.21 135.56 -0.25 0.17 2.13 84 107 82 TYR C, 200030 GLY B, 200092 ASN B, 84 VAL C, 200064 ARG C 3.28 135.82 -1.1 0.05 12.1 83 108 200030 GLY B, 200092 ASN B, 84 VAL C, 200064 ARG C, 200032 ASP B 3.1 137.07 -1.54 -0.38 21.72 92 109 200030 GLY B, 84 VAL C, 200064 ARG C, 200032 ASP B 2.94 138.24 -1.05 -0.28 26.3 89 110 200030 GLY B, 84 VAL C, 200064 ARG C 2.94 138.31 -0.23 -0.03 18.5 90 111 200030 GLY B, 84 VAL C, 200064 ARG C, 200031 THR B 2.94 138.42 -0.35 -0.22 14.29 96 112 200030 GLY B, 200064 ARG C, 200031 THR B 2.98 138.45 -1.87 -0.66 19.01 95 113 200030 GLY B, 200064 ARG C, 200032 ASP B, 200031 THR B 3 138.46 -2.28 -0.76 26.69 92 114 200030 GLY B, 200064 ARG C, 200032 ASP B, 200031 THR B 3.01 138.5 -2.2 -0.77 27.12 84 115 200030 GLY B, 200064 ARG C, 200031 THR B 3 138.56 -1.87 -0.66 19.01 95 116 84 VAL C, 200064 ARG C, 200032 ASP B, 200031 THR B 2.88 138.81 -1.13 -0.28 25.87 93 117 84 VAL C, 200064 ARG C, 200031 THR B 2.8 139.01 -0.33 -0.02 17.93 96 118 84 VAL C, 200064 ARG C, 200032 ASP B, 200031 THR B 2.6 139.64 -1.13 -0.28 25.87 93 119 84 VAL C, 200064 ARG C, 200031 THR B 2.51 140.11 -0.33 -0.02 17.93 96 120 84 VAL C, 200064 ARG C, 200032 ASP B, 200031 THR B 2.4 142.07 -1.13 -0.28 25.87 93 121 200064 ARG C, 200032 ASP B, 200031 THR B, 84 VAL C 2.46 142.75 -2.28 -0.76 26.69 92 122 200064 ARG C, 200032 ASP B, 200031 THR B, 84 VAL C, 200050 TYR B 2.56 143.85 -2.08 -0.38 21.67 81 123 200064 ARG C, 200031 THR B, 84 VAL C, 200050 TYR B 2.78 144.37 -1.73 -0.22 14.66 80 124 200064 ARG C, 200031 THR B, 200050 TYR B 2.88 144.47 -2.17 -0.03 18.42 80 125 200031 THR B, 200050 TYR B, 200053 GLU B 2.89 144.56 -1.83 -0.27 17.72 78 126 200031 THR B, 84 VAL C, 200050 TYR B, 200053 GLU B 2.89 146.2 -1.48 -0.4 14.14 78 127 200031 THR B, 84 VAL C, 200050 TYR B, 200053 GLU B, 85 THR C 3.08 146.63 -1.26 -0.48 11.99 78 128 200031 THR B, 84 VAL C, 200053 GLU B, 85 THR C 3.08 147.27 -1.33 -0.87 14.15 97 129 200031 THR B, 84 VAL C, 200053 GLU B, 85 THR C, 54 ALA C 3 148.37 -0.7 -0.69 11.32 97 130 200031 THR B, 200053 GLU B, 85 THR C, 54 ALA C 3.02 148.9 -0.78 -0.67 13.31 97 131 200053 GLU B, 85 THR C, 54 ALA C 2.99 150.15 -0.8 -0.63 17.19 94 132 200053 GLU B, 85 THR C, 54 ALA C, 53 GLY C 3 152.19 -0.7 -0.67 13.74 94 133 200053 GLU B, 85 THR C, 54 ALA C, 53 GLY C, 57 ARG A 3.54 152.8 -1.46 -0.62 21.39 91 134 200053 GLU B, 85 THR C, 53 GLY C, 57 ARG A 3.79 153.25 -2.28 -0.78 26.74 89 135 200053 GLU B, 53 GLY C, 57 ARG A 3.83 154.16 -2.8 -0.79 35.09 80 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
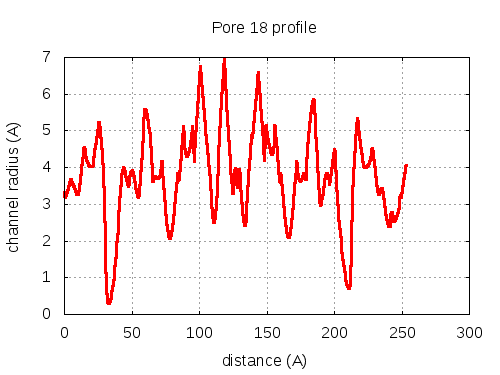
Unique lining residues set - all
100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B, 100155 VAL A, 100182 LEU A, 100171 PHE A, 100172 PRO A, 100153 GLU A, 100173 ALA A, 100172 PRO A, 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A, 100091 SER A, 100088 SER A, 100040 ARG A, 100091 SER A, 100043 HIS A, 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A, 100063 LYS A, 300020 SER B, 100003 LEU B, 100098 PHE B, 100061 ASN A, 100097 THR B, 100001 ASP B, 300072 THR B, 300070 ASP B, 100001 ASP B, 100026 SER B, 300024 ARG B, 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B, 300028 SER B, 100027 GLN B, 300028 SER B, 300027 GLN B, 100028 SER B, 27 GLN B, 28 SER B, 200027 GLN B, 200028 SER B, 100028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 300079 GLY C, 100079 GLY C, 93 ARG B, 79 GLY C, 82 TYR C, 200079 GLY C, 300082 TYR C, 200093 ARG B, 200082 TYR C, 100058 GLN C, 100080 ASP C, 100030 GLY B, 100092 ASN B, 200084 VAL C, 100064 ARG C, 100032 ASP B, 100031 THR B, 100031 THR B, 200084 VAL C, 100050 TYR B, 100053 GLU B, 200085 THR C, 200085 THR C, 200054 ALA C, 200053 GLY C, 200057 ARG A
Unique lining residues set - sidechains
100156 THR A, 100170 THR A, 100041 ASN B, 100182 LEU A, 100153 GLU A, 100172 PRO A, 100041 PRO A, 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A, 100040 ARG A, 100043 HIS A, 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A, 300020 SER B, 100003 LEU B, 100061 ASN A, 100097 THR B, 100001 ASP B, 300072 THR B, 300070 ASP B, 100026 SER B, 300024 ARG B, 300069 THR B, 100027 GLN B, 300028 SER B, 300027 GLN B, 27 GLN B, 200027 GLN B, 200028 SER B, 100028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 93 ARG B, 82 TYR C, 300082 TYR C, 200093 ARG B, 200082 TYR C, 100058 GLN C, 100092 ASN B, 200084 VAL C, 100064 ARG C, 100032 ASP B, 100031 THR B, 100050 TYR B, 100053 GLU B, 200085 THR C, 200054 ALA C, 200057 ARG A
Physicochemical properties of lining side-chains
Charge: 4 (10-6)
Hydropathy: -1.5
Hydrophobicity: -0.4
Polarity: 15.51
Mutability: 86
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B 3.28 0.26 -1.33 -0.78 2.52 106 2 100157 VAL A, 100170 THR A, 100041 ASN B 3.22 0.45 -1.53 -0.78 2.81 105 3 100170 THR A, 100041 ASN B, 100155 VAL A 3.19 0.62 -1.53 -0.78 2.81 105 4 100157 VAL A, 100170 THR A, 100041 ASN B 3.21 0.76 -1.53 -0.78 2.81 105 5 100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B 3.24 1.04 -1.33 -0.78 2.52 106 6 100156 THR A, 100157 VAL A, 100041 ASN B 3.31 1.57 -1.53 -0.78 2.81 105 7 100170 THR A, 100041 ASN B, 100155 VAL A 2.53 5.78 -1.53 -0.78 2.81 105 8 100170 THR A, 100041 ASN B, 100155 VAL A, 100182 LEU A 2.51 6.68 -0.2 -0.3 2.14 88 9 100170 THR A, 100041 ASN B, 100182 LEU A 2.77 6.86 -0.13 -0.13 1.72 88 10 100041 ASN B, 100182 LEU A, 100171 PHE A 2.76 7.22 -0.03 -0.14 2.3 79 11 100041 ASN B, 100182 LEU A, 100171 PHE A, 100172 PRO A 2.7 7.95 -0.13 -0.31 2.57 79 12 100041 ASN B, 100182 LEU A, 100171 PHE A, 100172 PRO A, 100153 GLU A 2.55 8.45 -0.8 -0.47 12.03 78 13 100041 ASN B, 100182 LEU A, 100172 PRO A, 100153 GLU A, 100173 ALA A 2.44 8.91 -0.8 -0.47 12.03 78 14 100041 ASN B, 100172 PRO A, 100153 GLU A, 100173 ALA A 2.28 10.1 -1.95 -0.88 15.01 90 15 100041 ASN B, 100153 GLU A, 100173 ALA A, 100172 PRO A 2.29 12.8 -2.25 -0.7 14.56 79 16 100041 ASN B, 100153 GLU A, 100172 PRO A 3.06 13.46 -2.87 -0.67 18.29 79 17 100041 ASN B, 100153 GLU A, 100172 PRO A, 100041 PRO A 3.3 13.73 -2.55 -0.52 14.11 74 18 100041 ASN B, 100153 GLU A, 100172 PRO A, 100041 PRO A 3.38 14.63 -2.55 -0.52 14.11 74 19 100153 GLU A, 100173 ALA A, 100172 PRO A, 100041 PRO A 3.36 16.89 -1.78 -0.53 14.11 64 20 100153 GLU A, 100173 ALA A, 100041 PRO A, 100180 TYR A 3.32 18.79 -1.7 -0.23 14.12 61 21 100173 ALA A, 100041 PRO A, 100180 TYR A 3.55 20.9 -1.1 0.07 2.19 54 22 100173 ALA A, 100041 PRO A, 100180 TYR A, 100175 LEU A 4.22 21.34 0.13 0.34 1.68 54 23 100041 PRO A, 100180 TYR A, 100175 LEU A 4.38 22.05 0.3 0.72 1.11 54 24 100173 ALA A, 100041 PRO A, 100180 TYR A, 100175 LEU A 4.49 22.37 0.13 0.34 1.68 54 25 100041 PRO A, 100180 TYR A, 100175 LEU A 4.03 27.04 0.3 0.72 1.11 54 26 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A, 100091 SER A 3.99 27.69 -0.06 0.08 1.67 69 27 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A 3.99 28.07 0.02 0.3 1.25 69 28 100180 TYR A, 100091 SER A, 100088 SER A 4.02 28.22 -0.97 -0.28 1.65 94 29 100091 SER A, 100088 SER A 4.07 28.29 -0.8 -0.97 1.67 117 30 100180 TYR A, 100091 SER A, 100088 SER A 4.15 28.65 -0.97 -0.28 1.65 94 31 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A 4.36 28.9 0.23 0.08 1.27 84 32 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A 4.47 29.24 0.02 0.3 1.25 69 33 100041 PRO A, 100175 LEU A, 100091 SER A, 100088 SER A 4.56 30.14 0.15 -0.22 1.26 86 34 100041 PRO A, 100091 SER A, 100088 SER A 4.72 30.63 -1.07 -0.68 1.64 97 35 100041 PRO A, 100088 SER A 4.82 31.82 -1.2 -0.53 1.63 87 36 100041 PRO A, 100088 SER A, 100040 ARG A 4.21 34.42 -2.3 -0.49 18.42 86 37 100041 PRO A, 100088 SER A, 100040 ARG A 3 36.56 -2.17 -0.44 18.99 70 38 100041 PRO A, 100088 SER A, 100040 ARG A, 100091 SER A 2.75 36.89 -1.73 -0.53 15.09 70 39 100088 SER A, 100040 ARG A, 100091 SER A 1.71 37.9 -1.77 -0.67 19.59 83 40 100088 SER A, 100040 ARG A 1.21 39.95 -2.45 -0.61 27.69 83 41 100040 ARG A 1.21 45.78 -4.5 -0.42 52 83 42 100040 ARG A, 100043 HIS A 2.68 46.83 -3.85 -0.08 51.8 87 43 100040 ARG A, 100046 GLU A 3.18 50.17 -4 -0.78 50.95 80 44 100040 ARG A, 100046 GLU A, 300018 ARG B 3.74 52.89 -4.17 -0.66 51.3 81 45 100040 ARG A, 100046 GLU A, 300018 ARG B, 100064 ILE A 3.52 56.19 -2 -0.04 38.51 86 46 100046 GLU A, 300018 ARG B, 100064 ILE A 3.87 56.78 -1.17 0.08 34.01 87 47 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A 3.59 59.48 -0.98 -0.14 26.35 87 48 100046 GLU A, 100064 ILE A, 100063 LYS A 3.26 60.28 0.2 -0.04 17.8 90 49 100046 GLU A, 100064 ILE A, 100063 LYS A 2.87 64.86 -0.97 0.09 33.18 84 50 100046 GLU A, 100064 ILE A, 100063 LYS A, 300020 SER B 5.17 65.32 -0.93 -0.18 25.3 92 51 100046 GLU A, 100063 LYS A, 300020 SER B, 100003 LEU B 5.31 66.29 -1.1 -0.35 25.3 80 52 100046 GLU A, 100064 ILE A, 100063 LYS A, 100003 LEU B 4.78 69.55 0.23 0.35 24.92 76 53 100046 GLU A, 100063 LYS A, 100003 LEU B, 100098 PHE B 4.52 70.51 -1 -0.3 25.73 67 54 100046 GLU A, 100063 LYS A, 100003 LEU B, 100098 PHE B, 100061 ASN A 4.3 71.14 -1.5 -0.4 21.26 76 55 100046 GLU A, 100063 LYS A, 100098 PHE B, 100061 ASN A, 100097 THR B 4.15 71.42 -2.4 -0.78 21.56 90 56 100046 GLU A, 100098 PHE B, 100061 ASN A, 100097 THR B 4.1 71.79 -2.03 -0.87 14.58 96 57 100046 GLU A, 100063 LYS A, 100098 PHE B, 100061 ASN A, 100097 THR B 4.2 72.01 -2.4 -0.78 21.56 90 58 100063 LYS A, 100003 LEU B, 100098 PHE B, 100061 ASN A, 100097 THR B 4.04 72.62 -0.94 -0.32 11.61 84 59 100063 LYS A, 100003 LEU B, 100098 PHE B, 100097 THR B 3.87 73.04 -0.3 -0.21 13.67 77 60 100063 LYS A, 100003 LEU B, 100061 ASN A, 100097 THR B 3.69 73.47 -1.08 -0.2 13.67 84 61 100063 LYS A, 100003 LEU B, 100097 THR B, 100001 ASP B 3.44 75.51 -1.08 -0.27 25.25 79 62 100063 LYS A, 100003 LEU B, 100001 ASP B 3.54 77.23 -1.2 -0.1 33.11 70 63 100063 LYS A, 100003 LEU B, 100001 ASP B, 300072 THR B 3.86 78.09 -1.08 -0.27 25.25 79 64 100063 LYS A, 100003 LEU B, 100001 ASP B, 300072 THR B, 300070 ASP B 4.07 78.29 -1.56 -0.42 30.14 81 65 100003 LEU B, 100001 ASP B, 300072 THR B, 300070 ASP B 3.95 79.18 -0.98 -0.43 25.3 83 66 100003 LEU B, 300070 ASP B, 100001 ASP B 3.04 81.49 -0.03 -0.23 17.74 70 67 100003 LEU B, 300070 ASP B 2.22 84.12 0.15 0.05 24.92 70 68 100003 LEU B, 300070 ASP B, 100026 SER B 2.12 87.09 -0.17 -0.29 17.17 85 69 100003 LEU B, 300070 ASP B, 100026 SER B, 300024 ARG B 2.58 88.82 -1.25 -0.32 25.88 85 70 300070 ASP B, 100026 SER B, 300024 ARG B 3.24 90.42 -2.93 -0.81 34.46 95 71 300070 ASP B, 300024 ARG B, 100026 SER B 3.63 91.47 -2.8 -0.75 35.03 84 72 300070 ASP B, 300024 ARG B, 100026 SER B, 300069 THR B 3.72 93 -2.28 -0.76 26.69 92 73 300024 ARG B, 100026 SER B, 300069 THR B 4.32 94.53 -1.87 -0.66 19.01 95 74 300024 ARG B, 100026 SER B, 300069 THR B, 300026 SER B 4.92 95.36 -1.5 -0.7 15.11 95 75 100026 SER B, 300069 THR B, 300026 SER B 4.32 98.25 -0.5 -0.79 2.81 107 76 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B 4.44 99.2 -0.48 -0.79 2.95 107 77 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B, 300028 SER B 4.65 101.31 -0.46 -0.79 3.04 107 78 100026 SER B, 300027 GLN B, 100027 GLN B, 300028 SER B 5.24 101.45 -1.28 -0.92 2.99 100 79 100026 SER B, 300069 THR B, 100027 GLN B, 300028 SER B 4.16 104.65 -1.35 -0.91 2.56 102 80 100026 SER B, 100027 GLN B, 300028 SER B 5.25 105.33 -1.57 -0.96 2.86 100 81 100027 GLN B, 300028 SER B, 300027 GLN B 5.54 105.95 -2.6 -1.06 2.91 95 82 100027 GLN B, 300028 SER B, 300027 GLN B, 100028 SER B 5.79 106.79 -2.05 -0.99 3.03 95 83 27 GLN B, 28 SER B, 200027 GLN B, 200028 SER B 6.18 107.8 -2.05 -0.99 3.03 95 84 100027 GLN B, 300028 SER B, 100028 SER B 4.89 111.32 -1.7 -1.01 2.29 106 85 100027 GLN B, 300028 SER B, 100028 SER B, 100093 ARG B 4.82 111.59 -2.4 -0.87 14.72 100 86 100027 GLN B, 100028 SER B, 100093 ARG B 4.82 112.02 -2.93 -0.83 19.07 94 87 100027 GLN B, 300028 SER B, 100028 SER B, 100093 ARG B 4.63 112.45 -2.4 -0.87 14.72 100 88 100027 GLN B, 300028 SER B, 100028 SER B, 100093 ARG B 4.36 113.06 -2.3 -0.82 15.15 94 89 100027 GLN B, 300028 SER B, 100093 ARG B 2.42 118.49 -2.93 -0.83 19.07 94 90 300028 SER B, 100093 ARG B, 100082 TYR C 3.16 120.25 -2.2 -0.09 18.43 83 91 300028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B 4.35 123.31 -2.78 -0.18 26.82 83 92 300028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 300079 GLY C 6.14 123.73 -2.3 -0.3 22.13 83 93 100093 ARG B, 100082 TYR C, 300093 ARG B, 300079 GLY C, 100079 GLY C 6.35 124.72 -2.22 -0.27 22.47 72 94 93 ARG B, 79 GLY C, 82 TYR C, 200079 GLY C, 300082 TYR C 6.51 124.95 -1.58 0.04 12.4 61 95 93 ARG B, 79 GLY C, 82 TYR C, 300082 TYR C, 200093 ARG B 6.49 125.36 -2.4 0.12 22.12 66 96 300093 ARG B, 93 ARG B, 79 GLY C, 82 TYR C, 300082 TYR C 6.48 125.83 -2.4 0.12 22.12 66 97 100093 ARG B, 100082 TYR C, 300079 GLY C, 100079 GLY C, 200082 TYR C 6.08 127.53 -1.58 0.04 12.4 61 98 100093 ARG B, 100082 TYR C, 100079 GLY C, 200082 TYR C 5.6 128.53 -1.88 0.25 14.65 61 99 100093 ARG B, 100082 TYR C, 100079 GLY C, 100058 GLN C 3.23 131.7 -2.43 -0.3 15.13 72 100 100093 ARG B, 100079 GLY C, 100058 GLN C, 100080 ASP C 3.48 132.08 -2.2 -0.78 15.57 83 101 100093 ARG B, 100079 GLY C, 200082 TYR C, 100058 GLN C 3.69 133.27 -2.43 -0.3 15.13 72 102 100093 ARG B, 200082 TYR C, 100058 GLN C, 100080 ASP C 3.71 133.48 -2.43 -0.3 15.13 72 103 100093 ARG B, 200082 TYR C, 100058 GLN C 3.57 134.56 -3.1 -0.14 19.05 72 104 100028 SER B, 100093 ARG B, 200082 TYR C, 100058 GLN C 3.59 134.78 -2.43 -0.3 15.13 72 105 100028 SER B, 100093 ARG B, 200082 TYR C, 100058 GLN C 3.62 135.15 -2.53 -0.35 14.7 83 106 100028 SER B, 100093 ARG B, 200082 TYR C 3.76 135.2 -2.2 -0.09 18.43 83 107 100028 SER B, 100093 ARG B, 200082 TYR C, 100058 GLN C 3.77 135.77 -2.53 -0.35 14.7 83 108 100028 SER B, 200082 TYR C, 100058 GLN C 3.38 138.1 -1.87 -0.32 2.27 83 109 100028 SER B, 200082 TYR C, 100058 GLN C, 100030 GLY B 3.15 139.26 -1.5 -0.44 2.55 83 110 200082 TYR C, 100058 GLN C, 100030 GLY B, 100092 ASN B 3.13 141.16 -2.18 -0.39 2.98 79 111 200082 TYR C, 100030 GLY B, 100092 ASN B 3.22 141.52 -1.73 -0.15 2.79 77 112 200082 TYR C, 100030 GLY B, 100092 ASN B, 200084 VAL C 3.28 142.37 -0.25 0.17 2.13 84 113 200082 TYR C, 100030 GLY B, 100092 ASN B, 200084 VAL C, 100064 ARG C 3.34 142.5 -1.1 0.05 12.1 83 114 100030 GLY B, 100092 ASN B, 200084 VAL C, 100064 ARG C, 100032 ASP B 3.07 143.8 -1.54 -0.38 21.72 92 115 100030 GLY B, 200084 VAL C, 100064 ARG C, 100032 ASP B 2.95 144.87 -1.05 -0.28 26.3 89 116 100030 GLY B, 200084 VAL C, 100064 ARG C 2.94 145.03 -0.23 -0.03 18.5 90 117 100030 GLY B, 200084 VAL C, 100064 ARG C, 100031 THR B 2.94 145.11 -0.35 -0.22 14.29 96 118 100030 GLY B, 100064 ARG C, 100031 THR B 2.95 145.14 -1.87 -0.66 19.01 95 119 100030 GLY B, 100064 ARG C, 100032 ASP B, 100031 THR B 2.97 145.16 -2.28 -0.76 26.69 92 120 100030 GLY B, 100064 ARG C, 100032 ASP B, 100031 THR B 3 145.19 -2.2 -0.77 27.12 84 121 100030 GLY B, 100064 ARG C, 100032 ASP B, 100031 THR B 3.02 145.25 -2.28 -0.76 26.69 92 122 100030 GLY B, 200084 VAL C, 100064 ARG C, 100031 THR B 3 145.34 -0.35 -0.22 14.29 96 123 200084 VAL C, 100064 ARG C, 100032 ASP B, 100031 THR B 2.93 145.49 -1.13 -0.28 25.87 93 124 200084 VAL C, 100064 ARG C, 100031 THR B 2.84 145.7 -0.33 -0.02 17.93 96 125 200084 VAL C, 100064 ARG C, 100032 ASP B, 100031 THR B 2.63 146.35 -1.13 -0.28 25.87 93 126 200084 VAL C, 100064 ARG C, 100031 THR B 2.55 146.84 -0.33 -0.02 17.93 96 127 200084 VAL C, 100064 ARG C, 100032 ASP B, 100031 THR B 2.49 148.91 -1.13 -0.28 25.87 93 128 100064 ARG C, 100032 ASP B, 100031 THR B, 200084 VAL C 2.51 149.62 -2.28 -0.76 26.69 92 129 100064 ARG C, 100032 ASP B, 100031 THR B, 200084 VAL C, 100050 TYR B 2.54 150.69 -2.08 -0.38 21.67 81 130 100064 ARG C, 100031 THR B, 200084 VAL C, 100050 TYR B 2.6 151.13 -1.73 -0.22 14.66 80 131 100031 THR B, 100050 TYR B, 100053 GLU B 2.73 151.35 -1.83 -0.27 17.72 78 132 100031 THR B, 200084 VAL C, 100050 TYR B, 100053 GLU B 2.92 152.79 -1.48 -0.4 14.14 78 133 100031 THR B, 200084 VAL C, 100050 TYR B, 100053 GLU B, 200085 THR C 3.13 153.24 -1.26 -0.48 11.99 78 134 100031 THR B, 200084 VAL C, 100053 GLU B, 200085 THR C 3.08 154.12 -1.33 -0.87 14.15 97 135 100031 THR B, 200084 VAL C, 100053 GLU B, 200085 THR C, 200054 ALA C 3 155.18 -0.7 -0.69 11.32 97 136 100031 THR B, 100053 GLU B, 200085 THR C, 200054 ALA C 2.93 155.76 -0.78 -0.67 13.31 97 137 100053 GLU B, 200085 THR C, 200054 ALA C 2.88 157.08 -0.8 -0.63 17.19 94 138 100053 GLU B, 200085 THR C, 200054 ALA C, 200053 GLY C 2.94 158.8 -0.7 -0.67 13.74 94 139 100053 GLU B, 200085 THR C, 200054 ALA C, 200053 GLY C, 200057 ARG A 3.53 159.46 -1.46 -0.62 21.39 91 140 100053 GLU B, 200085 THR C, 200053 GLY C, 200057 ARG A 3.8 159.91 -2.28 -0.78 26.74 89 141 100053 GLU B, 200053 GLY C, 200057 ARG A 3.83 160.86 -2.8 -0.79 35.09 80 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
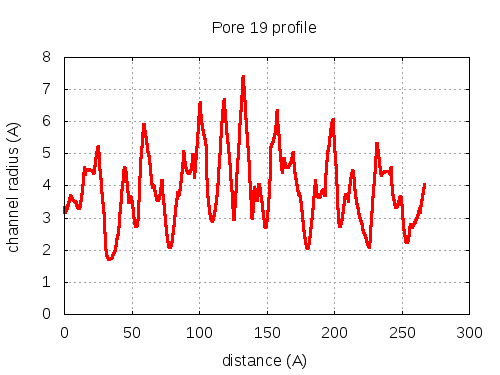
Unique lining residues set - all
100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B, 100155 VAL A, 100182 LEU A, 100171 PHE A, 100172 PRO A, 100153 GLU A, 100173 ALA A, 100172 PRO A, 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A, 100091 SER A, 100088 SER A, 100040 ARG A, 100091 SER A, 100043 HIS A, 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A, 100063 LYS A, 300020 SER B, 100003 LEU B, 100098 PHE B, 100061 ASN A, 100097 THR B, 100002 ILE B, 100001 ASP B, 300072 THR B, 300070 ASP B, 100001 ASP B, 100026 SER B, 300024 ARG B, 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B, 300028 SER B, 300028 SER B, 100027 GLN B, 300027 GLN B, 100028 SER B, 27 GLN B, 28 SER B, 200027 GLN B, 200028 SER B, 100028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 300079 GLY C, 100079 GLY C, 93 ARG B, 79 GLY C, 82 TYR C, 300082 TYR C, 200093 ARG B, 200079 GLY C, 200082 TYR C, 100058 GLN C, 100080 ASP C, 28 SER B, 200058 GLN C, 200080 ASP C, 200028 SER B, 200030 GLY B, 200092 ASN B, 84 VAL C, 200064 ARG C, 200032 ASP B, 200031 THR B, 200031 THR B, 84 VAL C, 200050 TYR B, 200053 GLU B, 85 THR C, 85 THR C, 54 ALA C, 53 GLY C, 57 ARG A
Unique lining residues set - sidechains
100156 THR A, 100170 THR A, 100041 ASN B, 100182 LEU A, 100153 GLU A, 100172 PRO A, 100041 PRO A, 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A, 100040 ARG A, 100043 HIS A, 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A, 300020 SER B, 100003 LEU B, 100061 ASN A, 100097 THR B, 100001 ASP B, 300072 THR B, 300070 ASP B, 100026 SER B, 300024 ARG B, 300069 THR B, 300028 SER B, 100027 GLN B, 300027 GLN B, 27 GLN B, 200027 GLN B, 200028 SER B, 100028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 93 ARG B, 82 TYR C, 300082 TYR C, 200093 ARG B, 200082 TYR C, 100058 GLN C, 28 SER B, 200058 GLN C, 200092 ASN B, 84 VAL C, 200064 ARG C, 200032 ASP B, 200031 THR B, 200050 TYR B, 200053 GLU B, 85 THR C, 54 ALA C, 57 ARG A
Physicochemical properties of lining side-chains
Charge: 4 (10-6)
Hydropathy: -1.6
Hydrophobicity: -0.39
Polarity: 15.95
Mutability: 86
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B 3.29 0.25 -1.33 -0.78 2.52 106 2 100157 VAL A, 100170 THR A, 100041 ASN B 3.23 0.43 -1.53 -0.78 2.81 105 3 100170 THR A, 100041 ASN B, 100155 VAL A 3.18 0.69 -1.53 -0.78 2.81 105 4 100157 VAL A, 100170 THR A, 100041 ASN B 3.2 0.85 -1.53 -0.78 2.81 105 5 100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B 3.19 0.99 -1.33 -0.78 2.52 106 6 100156 THR A, 100157 VAL A, 100041 ASN B 3.09 1.34 -1.53 -0.78 2.81 105 7 100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B, 100155 VAL A 3.03 1.8 -1.14 -0.78 2.69 106 8 100170 THR A, 100041 ASN B, 100155 VAL A 2.78 5.48 -1.53 -0.78 2.81 105 9 100170 THR A, 100041 ASN B, 100155 VAL A, 100182 LEU A 2.73 6.55 -0.2 -0.3 2.14 88 10 100170 THR A, 100041 ASN B, 100182 LEU A 2.76 6.87 -0.13 -0.13 1.72 88 11 100041 ASN B, 100182 LEU A, 100171 PHE A 2.75 7.23 -0.03 -0.14 2.3 79 12 100041 ASN B, 100182 LEU A, 100171 PHE A, 100172 PRO A 2.62 7.93 -0.13 -0.31 2.57 79 13 100041 ASN B, 100182 LEU A, 100171 PHE A, 100172 PRO A, 100153 GLU A 2.59 8.33 -0.8 -0.47 12.03 78 14 100041 ASN B, 100182 LEU A, 100172 PRO A, 100153 GLU A, 100173 ALA A 2.57 8.74 -0.8 -0.47 12.03 78 15 100041 ASN B, 100172 PRO A, 100153 GLU A, 100173 ALA A 2.51 9.85 -1.95 -0.88 15.01 90 16 100041 ASN B, 100153 GLU A, 100173 ALA A, 100172 PRO A 2.48 12.98 -2.25 -0.7 14.56 79 17 100041 ASN B, 100153 GLU A, 100172 PRO A 3.2 13.3 -2.87 -0.67 18.29 79 18 100041 ASN B, 100153 GLU A, 100172 PRO A, 100041 PRO A 3.39 13.83 -2.55 -0.52 14.11 74 19 100041 ASN B, 100153 GLU A, 100172 PRO A, 100041 PRO A 3.58 14.41 -2.55 -0.52 14.11 74 20 100153 GLU A, 100172 PRO A, 100041 PRO A 3.54 14.74 -2.23 -0.44 17.69 64 21 100153 GLU A, 100173 ALA A, 100172 PRO A, 100041 PRO A 3.28 16.97 -1.78 -0.53 14.11 64 22 100153 GLU A, 100173 ALA A, 100041 PRO A, 100180 TYR A 3.28 18.83 -1.7 -0.23 14.12 61 23 100173 ALA A, 100041 PRO A, 100180 TYR A 3.48 20.89 -1.1 0.07 2.19 54 24 100173 ALA A, 100041 PRO A, 100180 TYR A, 100175 LEU A 4.25 21.43 0.13 0.34 1.68 54 25 100041 PRO A, 100180 TYR A, 100175 LEU A 4.39 22.12 0.3 0.72 1.11 54 26 100173 ALA A, 100041 PRO A, 100180 TYR A, 100175 LEU A 4.47 22.55 0.13 0.34 1.68 54 27 100041 PRO A, 100180 TYR A, 100175 LEU A 4.05 27.33 0.3 0.72 1.11 54 28 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A, 100091 SER A 4.02 27.87 -0.06 0.08 1.67 69 29 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A 4.02 28.14 0.23 0.08 1.27 84 30 100180 TYR A, 100091 SER A, 100088 SER A 4.03 28.5 -0.97 -0.28 1.65 94 31 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A 4.21 28.97 0.23 0.08 1.27 84 32 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A 4.4 29.32 0.02 0.3 1.25 69 33 100041 PRO A, 100175 LEU A, 100091 SER A, 100088 SER A 4.5 29.74 0.15 -0.22 1.26 86 34 100041 PRO A, 100091 SER A, 100088 SER A 4.61 30.69 -1.07 -0.68 1.64 97 35 100041 PRO A, 100088 SER A 4.84 31.84 -1.2 -0.53 1.63 87 36 100041 PRO A, 100088 SER A, 100040 ARG A 4.42 34.34 -2.3 -0.49 18.42 86 37 100041 PRO A, 100088 SER A, 100040 ARG A 2.94 36.48 -2.17 -0.44 18.99 70 38 100041 PRO A, 100088 SER A, 100040 ARG A, 100091 SER A 2.77 36.82 -1.73 -0.53 15.09 70 39 100088 SER A, 100040 ARG A, 100091 SER A 1.44 38.4 -1.77 -0.67 19.59 83 40 100088 SER A, 100040 ARG A 0.84 39.46 -2.45 -0.61 27.69 83 41 100040 ARG A 0.02 45.19 -4.5 -0.42 52 83 42 100040 ARG A, 100043 HIS A 1.16 46.34 -3.85 -0.08 51.8 87 43 100040 ARG A, 100046 GLU A 1.98 50.43 -4 -0.78 50.95 80 44 100040 ARG A, 100046 GLU A, 300018 ARG B 4.19 52.49 -4.17 -0.66 51.3 81 45 100040 ARG A, 100046 GLU A, 300018 ARG B, 100064 ILE A 3.57 56.2 -2 -0.04 38.51 86 46 100046 GLU A, 300018 ARG B, 100064 ILE A 3.75 56.77 -1.17 0.08 34.01 87 47 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A 3.23 59.38 -0.98 -0.14 26.35 87 48 100046 GLU A, 100064 ILE A, 100063 LYS A 3.08 60.17 0.2 -0.04 17.8 90 49 100046 GLU A, 100064 ILE A, 100063 LYS A 3 64.66 -0.97 0.09 33.18 84 50 100046 GLU A, 100064 ILE A, 100063 LYS A, 300020 SER B 4.9 65.2 -0.93 -0.18 25.3 92 51 100046 GLU A, 100063 LYS A, 300020 SER B, 100003 LEU B 5.25 65.99 -1.1 -0.35 25.3 80 52 100046 GLU A, 100064 ILE A, 100063 LYS A, 100003 LEU B 4.86 70.07 0.23 0.35 24.92 76 53 100046 GLU A, 100063 LYS A, 100003 LEU B, 100098 PHE B 4.53 70.87 -1 -0.3 25.73 67 54 100046 GLU A, 100063 LYS A, 100003 LEU B, 100098 PHE B, 100061 ASN A 4.27 71.31 -1.5 -0.4 21.26 76 55 100046 GLU A, 100063 LYS A, 100098 PHE B, 100061 ASN A, 100097 THR B 4.14 71.47 -2.4 -0.78 21.56 90 56 100046 GLU A, 100098 PHE B, 100061 ASN A, 100097 THR B 4.11 71.68 -2.03 -0.87 14.58 96 57 100046 GLU A, 100063 LYS A, 100098 PHE B, 100061 ASN A, 100097 THR B 4.17 71.89 -2.4 -0.78 21.56 90 58 100063 LYS A, 100003 LEU B, 100098 PHE B, 100061 ASN A, 100097 THR B 3.94 72.76 -0.94 -0.32 11.61 84 59 100063 LYS A, 100003 LEU B, 100098 PHE B, 100097 THR B 3.78 73.17 -0.3 -0.21 13.67 77 60 100063 LYS A, 100003 LEU B, 100097 THR B, 100002 ILE B 3.63 73.6 -0.3 -0.21 13.67 77 61 100063 LYS A, 100003 LEU B, 100097 THR B, 100001 ASP B 3.46 75.62 -1.08 -0.27 25.25 79 62 100063 LYS A, 100003 LEU B, 100001 ASP B 3.56 77.28 -1.2 -0.1 33.11 70 63 100063 LYS A, 100003 LEU B, 100001 ASP B, 300072 THR B 3.84 78.11 -1.08 -0.27 25.25 79 64 100063 LYS A, 100003 LEU B, 100001 ASP B, 300072 THR B, 300070 ASP B 4.05 78.31 -1.56 -0.42 30.14 81 65 100003 LEU B, 100001 ASP B, 300072 THR B, 300070 ASP B 3.99 79.15 -0.98 -0.43 25.3 83 66 100003 LEU B, 300070 ASP B, 100001 ASP B 3.08 81.22 -0.03 -0.23 17.74 70 67 100003 LEU B, 300070 ASP B 2.12 84.26 0.15 0.05 24.92 70 68 100003 LEU B, 300070 ASP B, 100026 SER B 2.01 87.14 -0.17 -0.29 17.17 85 69 100003 LEU B, 300070 ASP B, 100026 SER B, 300024 ARG B 2.69 88.83 -1.25 -0.32 25.88 85 70 300070 ASP B, 100026 SER B, 300024 ARG B 3.41 90.44 -2.93 -0.81 34.46 95 71 300070 ASP B, 300024 ARG B, 100026 SER B 3.64 91.37 -2.8 -0.75 35.03 84 72 300070 ASP B, 300024 ARG B, 100026 SER B, 300069 THR B 3.71 93.33 -2.28 -0.76 26.69 92 73 300024 ARG B, 100026 SER B, 300069 THR B 4.37 94.69 -1.87 -0.66 19.01 95 74 300024 ARG B, 100026 SER B, 300069 THR B, 300026 SER B 4.79 95.5 -1.5 -0.7 15.11 95 75 100026 SER B, 300069 THR B, 300026 SER B 4.45 98.48 -0.5 -0.79 2.81 107 76 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B 4.64 99.39 -0.48 -0.79 2.95 107 77 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B, 300028 SER B 4.8 101.17 -0.46 -0.79 3.04 107 78 100026 SER B, 300069 THR B, 300027 GLN B, 300028 SER B 5.15 101.35 -0.58 -0.84 2.52 112 79 100026 SER B, 300027 GLN B, 300028 SER B, 100027 GLN B 5.16 101.49 -1.28 -0.92 2.99 100 80 100026 SER B, 300069 THR B, 300028 SER B, 100027 GLN B 4.16 104.53 -1.35 -0.91 2.56 102 81 100026 SER B, 300028 SER B, 100027 GLN B 5.18 105.19 -1.57 -0.96 2.86 100 82 300028 SER B, 100027 GLN B, 300027 GLN B 5.46 105.82 -2.6 -1.06 2.91 95 83 300028 SER B, 100027 GLN B, 300027 GLN B, 100028 SER B 5.71 106.73 -2.05 -0.99 3.03 95 84 27 GLN B, 28 SER B, 200027 GLN B, 200028 SER B 6.11 107.63 -2.05 -0.99 3.03 95 85 300028 SER B, 100027 GLN B, 100028 SER B 5.05 111.19 -1.7 -1.01 2.29 106 86 300028 SER B, 100027 GLN B, 100028 SER B, 100093 ARG B 4.87 111.55 -2.4 -0.87 14.72 100 87 100027 GLN B, 100028 SER B, 100093 ARG B 4.88 112.15 -2.93 -0.83 19.07 94 88 300028 SER B, 100027 GLN B, 100028 SER B, 100093 ARG B 4.63 112.61 -2.4 -0.87 14.72 100 89 300028 SER B, 100027 GLN B, 100028 SER B, 100093 ARG B 4.26 113.24 -2.3 -0.82 15.15 94 90 300028 SER B, 100027 GLN B, 100093 ARG B 2.52 117.81 -2.93 -0.83 19.07 94 91 300028 SER B, 100093 ARG B 2.73 118.54 -2.65 -0.7 26.84 100 92 300028 SER B, 100093 ARG B, 100082 TYR C 3 120.26 -2.2 -0.09 18.43 83 93 300028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B 4.09 123.25 -2.78 -0.18 26.82 83 94 300028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 300079 GLY C 6.08 124.04 -2.3 -0.3 22.13 83 95 100093 ARG B, 100082 TYR C, 300093 ARG B, 300079 GLY C, 100079 GLY C 6.6 124.66 -2.22 -0.27 22.47 72 96 300079 GLY C, 93 ARG B, 79 GLY C, 82 TYR C, 300082 TYR C 6.98 124.9 -1.58 0.04 12.4 61 97 93 ARG B, 79 GLY C, 82 TYR C, 200093 ARG B, 200079 GLY C 7.08 125.06 -2.22 -0.27 22.47 72 98 93 ARG B, 79 GLY C, 82 TYR C, 300082 TYR C, 200093 ARG B 7.02 125.24 -2.4 0.12 22.12 66 99 300093 ARG B, 93 ARG B, 79 GLY C, 82 TYR C, 300082 TYR C 6.83 125.57 -2.4 0.12 22.12 66 100 300093 ARG B, 300079 GLY C, 93 ARG B, 79 GLY C, 300082 TYR C 6.5 126.15 -2.22 -0.27 22.47 72 101 100093 ARG B, 100082 TYR C, 300079 GLY C, 100079 GLY C, 200082 TYR C 5.53 127.86 -1.58 0.04 12.4 61 102 100093 ARG B, 100082 TYR C, 100079 GLY C, 200082 TYR C 4.97 128.81 -1.88 0.25 14.65 61 103 100093 ARG B, 100082 TYR C, 100079 GLY C, 100058 GLN C 3.1 131.45 -2.43 -0.3 15.13 72 104 100093 ARG B, 100079 GLY C, 100058 GLN C 2.89 131.77 -2.8 -0.77 19.64 83 105 100093 ARG B, 100079 GLY C, 100058 GLN C, 100080 ASP C 2.83 133.11 -2.2 -0.78 15.57 83 106 100093 ARG B, 100079 GLY C, 200082 TYR C, 100058 GLN C 3.62 134.67 -2.43 -0.3 15.13 72 107 100093 ARG B, 100082 TYR C, 100079 GLY C, 200082 TYR C, 100058 GLN C 4.57 135.57 -2.2 -0.02 12.43 66 108 100093 ARG B, 100082 TYR C, 100079 GLY C, 200093 ARG B, 200082 TYR C 5.05 137.25 -2.4 0.12 22.12 66 109 100093 ARG B, 100079 GLY C, 200093 ARG B, 200079 GLY C, 200082 TYR C 5.92 138.34 -2.22 -0.27 22.47 72 110 300093 ARG B, 300079 GLY C, 93 ARG B, 79 GLY C, 300082 TYR C 6.63 138.72 -2.22 -0.27 22.47 72 111 300093 ARG B, 300079 GLY C, 93 ARG B, 300082 TYR C, 28 SER B 7.12 138.85 -2.3 -0.3 22.13 83 112 300093 ARG B, 300079 GLY C, 93 ARG B, 79 GLY C, 300082 TYR C 7.19 139.16 -2.22 -0.27 22.47 72 113 100093 ARG B, 300093 ARG B, 300079 GLY C, 93 ARG B, 300082 TYR C 7.09 139.71 -3.04 -0.19 32.2 74 114 100079 GLY C, 82 TYR C, 200093 ARG B, 200079 GLY C, 200082 TYR C 6.24 141.42 -1.58 0.04 12.4 61 115 82 TYR C, 200093 ARG B, 200079 GLY C, 200082 TYR C 5.54 142.39 -1.88 0.25 14.65 61 116 200093 ARG B, 200079 GLY C, 200082 TYR C, 200058 GLN C 3.27 145.49 -2.43 -0.3 15.13 72 117 200093 ARG B, 200079 GLY C, 200058 GLN C, 200080 ASP C 3.48 146.18 -2.2 -0.78 15.57 83 118 82 TYR C, 200093 ARG B, 200079 GLY C, 200058 GLN C 3.73 147.23 -2.43 -0.3 15.13 72 119 82 TYR C, 200093 ARG B, 200058 GLN C 3.56 148.26 -3.1 -0.14 19.05 72 120 82 TYR C, 200093 ARG B, 200058 GLN C, 200028 SER B 3.57 148.51 -2.43 -0.3 15.13 72 121 200028 SER B, 82 TYR C, 200093 ARG B, 200058 GLN C 3.6 149.73 -2.53 -0.35 14.7 83 122 200028 SER B, 82 TYR C, 200058 GLN C 3.39 152.05 -1.87 -0.32 2.27 83 123 200028 SER B, 82 TYR C, 200058 GLN C, 200030 GLY B 3.19 153.12 -1.5 -0.44 2.55 83 124 82 TYR C, 200058 GLN C, 200030 GLY B, 200092 ASN B 3.16 154.92 -2.18 -0.39 2.98 79 125 82 TYR C, 200030 GLY B, 200092 ASN B 3.16 155.59 -1.73 -0.15 2.79 77 126 82 TYR C, 200030 GLY B, 200092 ASN B, 84 VAL C 3.21 156.15 -0.25 0.17 2.13 84 127 82 TYR C, 200030 GLY B, 200092 ASN B, 84 VAL C, 200064 ARG C 3.25 156.43 -1.1 0.05 12.1 83 128 200030 GLY B, 200092 ASN B, 84 VAL C, 200064 ARG C, 200032 ASP B 3.09 157.81 -1.54 -0.38 21.72 92 129 200030 GLY B, 84 VAL C, 200064 ARG C, 200032 ASP B 2.98 158.74 -1.05 -0.28 26.3 89 130 200030 GLY B, 84 VAL C, 200064 ARG C 2.97 158.83 -0.23 -0.03 18.5 90 131 200030 GLY B, 84 VAL C, 200064 ARG C, 200031 THR B 2.96 158.95 -0.35 -0.22 14.29 96 132 200030 GLY B, 200064 ARG C, 200031 THR B 2.97 158.97 -1.87 -0.66 19.01 95 133 200030 GLY B, 200064 ARG C, 200032 ASP B, 200031 THR B 2.96 158.99 -2.28 -0.76 26.69 92 134 200030 GLY B, 200064 ARG C, 200032 ASP B, 200031 THR B 2.94 159.02 -2.2 -0.77 27.12 84 135 200030 GLY B, 200064 ARG C, 200031 THR B 2.92 159.08 -1.87 -0.66 19.01 95 136 84 VAL C, 200064 ARG C, 200032 ASP B, 200031 THR B 2.84 159.34 -1.13 -0.28 25.87 93 137 84 VAL C, 200064 ARG C, 200031 THR B 2.78 159.54 -0.33 -0.02 17.93 96 138 84 VAL C, 200064 ARG C, 200032 ASP B, 200031 THR B 2.62 160.19 -1.13 -0.28 25.87 93 139 84 VAL C, 200064 ARG C, 200031 THR B 2.54 160.68 -0.33 -0.02 17.93 96 140 84 VAL C, 200064 ARG C, 200032 ASP B, 200031 THR B 2.43 162.7 -1.13 -0.28 25.87 93 141 200064 ARG C, 200032 ASP B, 200031 THR B, 84 VAL C 2.49 163.39 -2.28 -0.76 26.69 92 142 200064 ARG C, 200032 ASP B, 200031 THR B, 84 VAL C, 200050 TYR B 2.57 164.47 -2.08 -0.38 21.67 81 143 200064 ARG C, 200031 THR B, 84 VAL C, 200050 TYR B 2.76 164.94 -1.73 -0.22 14.66 80 144 200064 ARG C, 200031 THR B, 200050 TYR B 2.9 165.02 -2.17 -0.03 18.42 80 145 200031 THR B, 200050 TYR B, 200053 GLU B 2.95 165.13 -1.83 -0.27 17.72 78 146 200031 THR B, 84 VAL C, 200050 TYR B, 200053 GLU B 2.98 166.46 -1.48 -0.4 14.14 78 147 200031 THR B, 84 VAL C, 200050 TYR B, 200053 GLU B, 85 THR C 3.11 166.91 -1.26 -0.48 11.99 78 148 200031 THR B, 84 VAL C, 200053 GLU B, 85 THR C 3.13 167.88 -1.33 -0.87 14.15 97 149 200031 THR B, 84 VAL C, 200053 GLU B, 85 THR C, 54 ALA C 2.88 169.22 -0.7 -0.69 11.32 97 150 200031 THR B, 200053 GLU B, 85 THR C, 54 ALA C 2.84 169.83 -0.78 -0.67 13.31 97 151 200053 GLU B, 85 THR C, 54 ALA C 2.85 171.11 -0.8 -0.63 17.19 94 152 200053 GLU B, 85 THR C, 54 ALA C, 53 GLY C 3.02 172.67 -0.7 -0.67 13.74 94 153 200053 GLU B, 85 THR C, 54 ALA C, 53 GLY C, 57 ARG A 3.58 173.31 -1.46 -0.62 21.39 91 154 200053 GLU B, 85 THR C, 53 GLY C, 57 ARG A 3.81 173.76 -2.28 -0.78 26.74 89 155 200053 GLU B, 53 GLY C, 57 ARG A 3.84 174.69 -2.8 -0.79 35.09 80 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
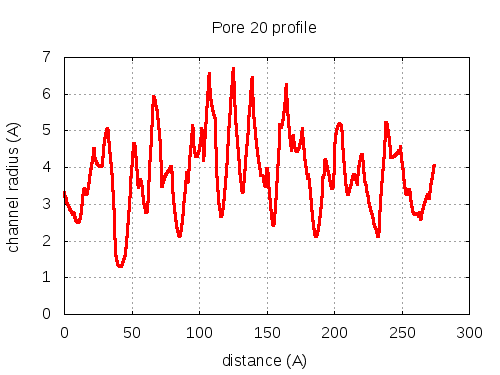
Unique lining residues set - all
100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B, 100155 VAL A, 100182 LEU A, 100171 PHE A, 100172 PRO A, 100153 GLU A, 100173 ALA A, 100172 PRO A, 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A, 100091 SER A, 100088 SER A, 100040 ARG A, 100091 SER A, 100043 HIS A, 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A, 100063 LYS A, 300020 SER B, 100003 LEU B, 100098 PHE B, 100061 ASN A, 100097 THR B, 100001 ASP B, 300072 THR B, 300070 ASP B, 100001 ASP B, 100026 SER B, 300024 ARG B, 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B, 300028 SER B, 100027 GLN B, 300028 SER B, 100028 SER B, 300027 GLN B, 27 GLN B, 28 SER B, 200027 GLN B, 200028 SER B, 100028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 300058 GLN C, 300079 GLY C, 300080 ASP C, 300082 TYR C, 79 GLY C, 93 ARG B, 200079 GLY C, 200093 ARG B, 82 TYR C, 200082 TYR C, 100079 GLY C, 28 SER B, 200028 SER B, 69 THR B, 27 GLN B, 26 SER B, 24 ARG B, 70 ASP B, 300026 SER B, 300003 LEU B, 300001 ASP B, 72 THR B, 300001 ASP B, 300063 LYS A, 300097 THR B, 300002 ILE B, 300061 ASN A, 300098 PHE B, 300046 GLU A, 300064 ILE A, 20 SER B, 300063 LYS A, 18 ARG B, 300040 ARG A, 300088 SER A, 300091 SER A, 300091 SER A, 300041 PRO A, 300088 SER A, 300175 LEU A, 300180 TYR A, 300173 ALA A, 300153 GLU A, 300172 PRO A, 300041 ASN B, 300042 GLY A, 300165 ASP B, 300040 THR B, 300085 ASN B, 300103 LYS B, 300010 ILE B
Unique lining residues set - sidechains
100156 THR A, 100170 THR A, 100041 ASN B, 100182 LEU A, 100153 GLU A, 100172 PRO A, 100041 PRO A, 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A, 100040 ARG A, 100043 HIS A, 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A, 300020 SER B, 100003 LEU B, 100061 ASN A, 100097 THR B, 100001 ASP B, 300072 THR B, 300070 ASP B, 100026 SER B, 300024 ARG B, 300069 THR B, 100027 GLN B, 300028 SER B, 300027 GLN B, 27 GLN B, 200027 GLN B, 200028 SER B, 100028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 300058 GLN C, 300082 TYR C, 93 ARG B, 200093 ARG B, 82 TYR C, 200082 TYR C, 28 SER B, 69 THR B, 24 ARG B, 70 ASP B, 300026 SER B, 300003 LEU B, 72 THR B, 300001 ASP B, 300063 LYS A, 300097 THR B, 300061 ASN A, 300046 GLU A, 300064 ILE A, 20 SER B, 18 ARG B, 300040 ARG A, 300091 SER A, 300041 PRO A, 300088 SER A, 300175 LEU A, 300180 TYR A, 300153 GLU A, 300172 PRO A, 300041 ASN B, 300165 ASP B, 300040 THR B, 300085 ASN B, 300103 LYS B, 300010 ILE B
Physicochemical properties of lining side-chains
Charge: 4 (13-9)
Hydropathy: -1.5
Hydrophobicity: -0.43
Polarity: 15.04
Mutability: 87
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B 3.26 0.26 -1.33 -0.78 2.52 106 2 100157 VAL A, 100170 THR A, 100041 ASN B 3.2 0.45 -1.53 -0.78 2.81 105 3 100170 THR A, 100041 ASN B, 100155 VAL A 3.2 0.61 -1.53 -0.78 2.81 105 4 100157 VAL A, 100170 THR A, 100041 ASN B 3.22 0.75 -1.53 -0.78 2.81 105 5 100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B 3.2 1.03 -1.33 -0.78 2.52 106 6 100156 THR A, 100157 VAL A, 100041 ASN B 3 1.52 -1.53 -0.78 2.81 105 7 100157 VAL A, 100170 THR A, 100041 ASN B, 100155 VAL A 2.89 2.1 -1.25 -0.79 2.95 105 8 100170 THR A, 100041 ASN B, 100155 VAL A 2.51 5.74 -1.53 -0.78 2.81 105 9 100170 THR A, 100041 ASN B, 100155 VAL A, 100182 LEU A 2.52 6.67 -0.2 -0.3 2.14 88 10 100170 THR A, 100041 ASN B, 100182 LEU A 2.78 7.03 -0.13 -0.13 1.72 88 11 100041 ASN B, 100182 LEU A, 100171 PHE A 2.76 7.21 -0.03 -0.14 2.3 79 12 100041 ASN B, 100182 LEU A, 100171 PHE A, 100172 PRO A 2.56 7.94 -0.13 -0.31 2.57 79 13 100041 ASN B, 100182 LEU A, 100171 PHE A, 100172 PRO A, 100153 GLU A 2.4 8.36 -0.8 -0.47 12.03 78 14 100041 ASN B, 100182 LEU A, 100172 PRO A, 100153 GLU A, 100173 ALA A 2.34 8.79 -0.8 -0.47 12.03 78 15 100041 ASN B, 100172 PRO A, 100153 GLU A, 100173 ALA A 2.31 9.93 -1.95 -0.88 15.01 90 16 100041 ASN B, 100153 GLU A, 100173 ALA A, 100172 PRO A 2.38 12.69 -2.25 -0.7 14.56 79 17 100041 ASN B, 100153 GLU A, 100172 PRO A 3.13 13.39 -2.87 -0.67 18.29 79 18 100041 ASN B, 100153 GLU A, 100172 PRO A, 100041 PRO A 3.37 13.92 -2.55 -0.52 14.11 74 19 100041 ASN B, 100153 GLU A, 100172 PRO A, 100041 PRO A 3.48 14.53 -2.55 -0.52 14.11 74 20 100153 GLU A, 100173 ALA A, 100172 PRO A, 100041 PRO A 3.31 16.74 -1.78 -0.53 14.11 64 21 100153 GLU A, 100173 ALA A, 100041 PRO A, 100180 TYR A 3.3 19.15 -1.7 -0.23 14.12 61 22 100173 ALA A, 100041 PRO A, 100180 TYR A 3.77 21.07 -1.1 0.07 2.19 54 23 100173 ALA A, 100041 PRO A, 100180 TYR A, 100175 LEU A 4.44 21.42 0.13 0.34 1.68 54 24 100041 PRO A, 100180 TYR A, 100175 LEU A 4.37 22.12 0.3 0.72 1.11 54 25 100173 ALA A, 100041 PRO A, 100180 TYR A, 100175 LEU A 4.45 22.54 0.13 0.34 1.68 54 26 100041 PRO A, 100180 TYR A, 100175 LEU A 4.21 27.38 0.3 0.72 1.11 54 27 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A, 100091 SER A 4.2 27.91 -0.06 0.08 1.67 69 28 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A 4.21 28.17 0.23 0.08 1.27 84 29 100091 SER A, 100088 SER A 4.23 28.26 -0.8 -0.97 1.67 117 30 100180 TYR A, 100091 SER A, 100088 SER A 4.28 28.55 -0.97 -0.28 1.65 94 31 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A 4.42 29.05 0.23 0.08 1.27 84 32 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A 4.62 29.42 0.02 0.3 1.25 69 33 100041 PRO A, 100175 LEU A, 100091 SER A, 100088 SER A 4.73 29.87 0.15 -0.22 1.26 86 34 100041 PRO A, 100091 SER A, 100088 SER A 4.83 30.84 -1.07 -0.68 1.64 97 35 100041 PRO A, 100088 SER A 5.01 31.65 -1.2 -0.53 1.63 87 36 100041 PRO A, 100088 SER A, 100040 ARG A 4.52 34 -2.3 -0.49 18.42 86 37 100041 PRO A, 100088 SER A, 100040 ARG A 3.55 36.32 -2.17 -0.44 18.99 70 38 100041 PRO A, 100088 SER A, 100040 ARG A, 100091 SER A 2.83 37.01 -1.73 -0.53 15.09 70 39 100088 SER A, 100040 ARG A, 100091 SER A 1.4 38.13 -1.77 -0.67 19.59 83 40 100088 SER A, 100040 ARG A 0.93 39.08 -2.45 -0.61 27.69 83 41 100040 ARG A 0.22 44.87 -4.5 -0.42 52 83 42 100040 ARG A, 100043 HIS A 0.79 46.11 -3.85 -0.08 51.8 87 43 100040 ARG A, 100046 GLU A 1.4 50.36 -4 -0.78 50.95 80 44 100040 ARG A, 100046 GLU A, 300018 ARG B 4.62 52.47 -4.17 -0.66 51.3 81 45 100040 ARG A, 100046 GLU A, 300018 ARG B, 100064 ILE A 3.53 56.3 -2 -0.04 38.51 86 46 100046 GLU A, 300018 ARG B, 100064 ILE A 3.49 56.89 -1.17 0.08 34.01 87 47 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A 3.15 59.6 -0.98 -0.14 26.35 87 48 100046 GLU A, 100064 ILE A, 100063 LYS A 3.21 60.39 0.2 -0.04 17.8 90 49 100046 GLU A, 100064 ILE A, 100063 LYS A 3.37 64.93 -0.97 0.09 33.18 84 50 100046 GLU A, 100064 ILE A, 100063 LYS A, 300020 SER B 4.97 65.36 -0.93 -0.18 25.3 92 51 100046 GLU A, 100063 LYS A, 300020 SER B, 100003 LEU B 4.8 66.36 -1.1 -0.35 25.3 80 52 100046 GLU A, 100064 ILE A, 100063 LYS A, 100003 LEU B 4.32 69.62 0.23 0.35 24.92 76 53 100046 GLU A, 100063 LYS A, 100003 LEU B, 100098 PHE B 4.21 70.57 -1 -0.3 25.73 67 54 100046 GLU A, 100063 LYS A, 100003 LEU B, 100098 PHE B, 100061 ASN A 4.15 71.17 -1.5 -0.4 21.26 76 55 100046 GLU A, 100063 LYS A, 100098 PHE B, 100061 ASN A, 100097 THR B 4.14 71.44 -2.4 -0.78 21.56 90 56 100046 GLU A, 100098 PHE B, 100061 ASN A, 100097 THR B 4.16 71.6 -2.03 -0.87 14.58 96 57 100046 GLU A, 100063 LYS A, 100098 PHE B, 100061 ASN A, 100097 THR B 4.2 72.03 -2.4 -0.78 21.56 90 58 100063 LYS A, 100003 LEU B, 100098 PHE B, 100061 ASN A, 100097 THR B 4.07 72.64 -0.94 -0.32 11.61 84 59 100063 LYS A, 100003 LEU B, 100098 PHE B, 100097 THR B 3.97 73.05 -0.3 -0.21 13.67 77 60 100063 LYS A, 100003 LEU B, 100061 ASN A, 100097 THR B 3.84 73.49 -1.08 -0.2 13.67 84 61 100063 LYS A, 100003 LEU B, 100097 THR B, 100001 ASP B 3.56 75.51 -1.08 -0.27 25.25 79 62 100063 LYS A, 100003 LEU B, 100001 ASP B 3.6 77.23 -1.2 -0.1 33.11 70 63 100063 LYS A, 100003 LEU B, 100001 ASP B, 300072 THR B 3.86 78.09 -1.08 -0.27 25.25 79 64 100063 LYS A, 100003 LEU B, 100001 ASP B, 300072 THR B, 300070 ASP B 4.11 78.29 -1.56 -0.42 30.14 81 65 100003 LEU B, 100001 ASP B, 300072 THR B, 300070 ASP B 3.83 79.17 -0.98 -0.43 25.3 83 66 100003 LEU B, 300070 ASP B, 100001 ASP B 3.04 81.41 -0.03 -0.23 17.74 70 67 100003 LEU B, 300070 ASP B 2.18 84.53 0.15 0.05 24.92 70 68 100003 LEU B, 300070 ASP B, 100026 SER B 2.09 86.98 -0.17 -0.29 17.17 85 69 100003 LEU B, 300070 ASP B, 100026 SER B, 300024 ARG B 2.46 88.72 -1.25 -0.32 25.88 85 70 300070 ASP B, 100026 SER B, 300024 ARG B 3.22 90.43 -2.93 -0.81 34.46 95 71 300070 ASP B, 300024 ARG B, 100026 SER B 3.57 91.37 -2.8 -0.75 35.03 84 72 300070 ASP B, 300024 ARG B, 100026 SER B, 300069 THR B 3.73 93.39 -2.28 -0.76 26.69 92 73 300024 ARG B, 100026 SER B, 300069 THR B 4.66 94.41 -1.87 -0.66 19.01 95 74 300024 ARG B, 100026 SER B, 300069 THR B, 300026 SER B 4.62 95.6 -1.5 -0.7 15.11 95 75 100026 SER B, 300069 THR B, 300026 SER B 4.47 98.81 -0.5 -0.79 2.81 107 76 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B 4.76 99.71 -0.48 -0.79 2.95 107 77 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B, 300028 SER B 4.92 101.25 -0.46 -0.79 3.04 107 78 100026 SER B, 300027 GLN B, 100027 GLN B, 300028 SER B 5.07 101.56 -1.28 -0.92 2.99 100 79 100026 SER B, 300069 THR B, 100027 GLN B, 300028 SER B 4.24 104.32 -1.35 -0.91 2.56 102 80 100026 SER B, 100027 GLN B, 300028 SER B 5.26 104.99 -1.57 -0.96 2.86 100 81 300027 GLN B, 100027 GLN B, 300028 SER B 5.55 105.65 -1.57 -0.96 2.86 100 82 100027 GLN B, 300028 SER B, 100028 SER B, 300027 GLN B 5.83 106.64 -2.05 -0.99 3.03 95 83 27 GLN B, 28 SER B, 200027 GLN B, 200028 SER B 6.29 107.56 -2.05 -0.99 3.03 95 84 100027 GLN B, 300028 SER B, 100028 SER B 5.1 111.17 -1.7 -1.01 2.29 106 85 100027 GLN B, 300028 SER B, 100028 SER B, 100093 ARG B 4.81 111.54 -2.4 -0.87 14.72 100 86 100027 GLN B, 100028 SER B, 100093 ARG B 4.71 112.2 -2.93 -0.83 19.07 94 87 100027 GLN B, 300028 SER B, 100028 SER B, 100093 ARG B 4.62 112.72 -2.4 -0.87 14.72 100 88 100027 GLN B, 300028 SER B, 100028 SER B, 100093 ARG B 4.44 113.4 -2.3 -0.82 15.15 94 89 100027 GLN B, 300028 SER B, 100093 ARG B 2.48 118.63 -2.93 -0.83 19.07 94 90 300028 SER B, 100093 ARG B, 100082 TYR C 2.7 120.24 -2.2 -0.09 18.43 83 91 300028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B 3.48 120.61 -2.78 -0.18 26.82 83 92 300028 SER B, 100082 TYR C, 300093 ARG B, 300058 GLN C 3.71 121.64 -2.53 -0.35 14.7 83 93 300028 SER B, 300093 ARG B, 300058 GLN C 3.64 121.92 -2.93 -0.83 19.07 94 94 300028 SER B, 100082 TYR C, 300093 ARG B, 300058 GLN C 3.52 122.52 -2.53 -0.35 14.7 83 95 300028 SER B, 100082 TYR C, 300093 ARG B, 300058 GLN C 3.52 122.79 -2.43 -0.3 15.13 72 96 100082 TYR C, 300093 ARG B, 300058 GLN C 3.54 123.69 -3.1 -0.14 19.05 72 97 100082 TYR C, 300093 ARG B, 300058 GLN C, 300079 GLY C 3.71 124.95 -2.43 -0.3 15.13 72 98 300093 ARG B, 300058 GLN C, 300079 GLY C, 300080 ASP C 3.73 125.64 -2.2 -0.78 15.57 83 99 300093 ARG B, 300058 GLN C, 300079 GLY C, 300082 TYR C 3.69 128.65 -2.43 -0.3 15.13 72 100 100082 TYR C, 300093 ARG B, 300079 GLY C, 300082 TYR C 4.43 129.65 -1.88 0.25 14.65 61 101 100082 TYR C, 300093 ARG B, 300079 GLY C, 300082 TYR C, 79 GLY C 4.81 131.23 -1.58 0.04 12.4 61 102 300093 ARG B, 300079 GLY C, 79 GLY C, 93 ARG B, 200079 GLY C 5.65 131.63 -2.04 -0.65 22.83 83 103 93 ARG B, 200079 GLY C, 200093 ARG B, 82 TYR C, 200082 TYR C 6.03 131.78 -2.4 0.12 22.12 66 104 100093 ARG B, 200079 GLY C, 200093 ARG B, 82 TYR C, 200082 TYR C 6.34 132.25 -2.4 0.12 22.12 66 105 200079 GLY C, 200093 ARG B, 82 TYR C, 200082 TYR C, 100079 GLY C 6.63 132.55 -1.58 0.04 12.4 61 106 300093 ARG B, 300079 GLY C, 300082 TYR C, 79 GLY C, 93 ARG B 6.41 133.28 -2.22 -0.27 22.47 72 107 300093 ARG B, 300082 TYR C, 79 GLY C, 93 ARG B, 28 SER B 6.01 134.13 -2.3 -0.3 22.13 83 108 300093 ARG B, 300082 TYR C, 93 ARG B, 28 SER B 4.17 137.23 -2.78 -0.18 26.82 83 109 300093 ARG B, 300082 TYR C, 28 SER B 3.44 138.54 -2.2 -0.09 18.43 83 110 300093 ARG B, 28 SER B 3.08 139.29 -2.65 -0.7 26.84 100 111 300027 GLN B, 300093 ARG B, 28 SER B 2.47 144.53 -2.93 -0.83 19.07 94 112 300028 SER B, 300027 GLN B, 300093 ARG B, 28 SER B 4.34 145.11 -2.4 -0.87 14.72 100 113 300028 SER B, 300027 GLN B, 300093 ARG B 4.97 145.45 -2.93 -0.83 19.07 94 114 300028 SER B, 300027 GLN B, 300093 ARG B, 28 SER B 5.1 145.96 -2.4 -0.87 14.72 100 115 300028 SER B, 300027 GLN B, 28 SER B 5.07 149.55 -1.7 -1.01 2.29 106 116 100027 GLN B, 200027 GLN B, 100028 SER B, 200028 SER B 6 150.46 -2.05 -0.99 3.03 95 117 300028 SER B, 300027 GLN B, 27 GLN B, 28 SER B 5.6 151.59 -2.05 -0.99 3.03 95 118 300027 GLN B, 27 GLN B, 28 SER B 5.33 152.27 -2.6 -1.06 2.91 95 119 300026 SER B, 300027 GLN B, 28 SER B 5.07 152.92 -1.57 -0.96 2.86 100 120 300026 SER B, 300027 GLN B, 28 SER B, 69 THR B 4.51 155.59 -1.35 -0.91 2.56 102 121 300026 SER B, 300027 GLN B, 28 SER B, 27 GLN B 4.85 155.78 -1.28 -0.92 2.99 100 122 300026 SER B, 28 SER B, 69 THR B, 27 GLN B 4.9 156.17 -0.58 -0.84 2.52 112 123 300026 SER B, 28 SER B, 69 THR B, 27 GLN B, 26 SER B 4.94 158.58 -0.46 -0.79 3.04 107 124 300026 SER B, 69 THR B, 27 GLN B, 26 SER B 5 159.52 -0.48 -0.79 2.95 107 125 300026 SER B, 69 THR B, 26 SER B 4.96 161.8 -0.5 -0.79 2.81 107 126 300026 SER B, 69 THR B, 26 SER B, 24 ARG B 4.71 162.73 -1.5 -0.7 15.11 95 127 300026 SER B, 69 THR B, 24 ARG B 4.39 164.41 -1.87 -0.66 19.01 95 128 300026 SER B, 69 THR B, 24 ARG B, 70 ASP B 3.96 165.7 -2.28 -0.76 26.69 92 129 300026 SER B, 24 ARG B, 70 ASP B 3.63 166.56 -2.8 -0.75 35.03 84 130 24 ARG B, 70 ASP B, 300026 SER B 3.49 168.42 -2.93 -0.81 34.46 95 131 24 ARG B, 70 ASP B, 300026 SER B, 300003 LEU B 2.63 170.68 -1.25 -0.32 25.88 85 132 70 ASP B, 300026 SER B, 300003 LEU B 2.09 173.15 -0.17 -0.29 17.17 85 133 70 ASP B, 300003 LEU B 2.15 176.15 0.15 0.05 24.92 70 134 70 ASP B, 300003 LEU B, 300001 ASP B 3.06 177.83 -0.03 -0.23 17.74 70 135 70 ASP B, 300003 LEU B, 300001 ASP B, 72 THR B 3.92 177.96 -0.2 -0.37 13.72 82 136 70 ASP B, 300003 LEU B, 72 THR B, 300001 ASP B 3.95 178.93 -0.98 -0.43 25.3 83 137 70 ASP B, 300003 LEU B, 72 THR B, 300001 ASP B, 300063 LYS A 4.09 179.16 -1.56 -0.42 30.14 81 138 300003 LEU B, 72 THR B, 300001 ASP B, 300063 LYS A 3.99 180.48 -1.08 -0.27 25.25 79 139 300003 LEU B, 300001 ASP B, 300063 LYS A 3.75 182.27 -1.2 -0.1 33.11 70 140 300003 LEU B, 300001 ASP B, 300063 LYS A, 300097 THR B 3.52 183.65 -1.08 -0.27 25.25 79 141 300003 LEU B, 300063 LYS A, 300097 THR B, 300002 ILE B 3.49 184.08 -0.3 -0.21 13.67 77 142 300003 LEU B, 300063 LYS A, 300097 THR B, 300061 ASN A 3.49 184.46 -1.08 -0.2 13.67 84 143 300003 LEU B, 300063 LYS A, 300097 THR B, 300061 ASN A, 300098 PHE B 3.51 185.22 -0.94 -0.32 11.61 84 144 300063 LYS A, 300097 THR B, 300061 ASN A, 300098 PHE B, 300046 GLU A 3.8 185.4 -2.4 -0.78 21.56 90 145 300097 THR B, 300061 ASN A, 300098 PHE B, 300046 GLU A 3.96 185.6 -2.03 -0.87 14.58 96 146 300063 LYS A, 300097 THR B, 300061 ASN A, 300098 PHE B, 300046 GLU A 4.39 186.02 -2.4 -0.78 21.56 90 147 300003 LEU B, 300063 LYS A, 300061 ASN A, 300098 PHE B, 300046 GLU A 4.61 187.86 -1.5 -0.4 21.26 76 148 300003 LEU B, 300063 LYS A, 300046 GLU A, 300064 ILE A 4.87 191.46 0.23 0.35 24.92 76 149 300003 LEU B, 300063 LYS A, 300046 GLU A, 20 SER B 4.42 192.33 -1.1 -0.35 25.3 80 150 300063 LYS A, 300046 GLU A, 300064 ILE A 3.54 197.74 -0.97 0.09 33.18 84 151 300046 GLU A, 300064 ILE A, 300063 LYS A, 18 ARG B 3.83 200.93 -0.98 -0.14 26.35 87 152 300046 GLU A, 300064 ILE A, 18 ARG B, 300040 ARG A 3.53 204.72 -2 -0.04 38.51 86 153 300046 GLU A, 18 ARG B, 300040 ARG A 4.1 206.92 -4.17 -0.66 51.3 81 154 300046 GLU A, 300064 ILE A, 300040 ARG A 4.17 207.39 -1.17 0.08 34.01 87 155 300046 GLU A, 300040 ARG A 2.11 210.69 -4 -0.78 50.95 80 156 300040 ARG A -0.04 216.9 -4.5 -0.42 52 83 157 300040 ARG A, 300088 SER A 0.02 217.75 -2.45 -0.61 27.69 83 158 300040 ARG A, 300088 SER A, 300091 SER A 0.31 218.22 -1.77 -0.67 19.59 83 159 300040 ARG A, 300088 SER A, 300091 SER A 0.78 218.48 -1.9 -0.73 19.02 100 160 300040 ARG A, 300088 SER A, 300091 SER A, 300041 PRO A 1.37 218.72 -1.83 -0.57 14.66 86 161 300040 ARG A, 300088 SER A, 300091 SER A, 300041 PRO A 2.01 219.43 -1.73 -0.53 15.09 70 162 300040 ARG A, 300088 SER A, 300041 PRO A 3.26 221.88 -2.17 -0.44 18.99 70 163 300040 ARG A, 300041 PRO A, 300088 SER A 4.87 224.06 -2.3 -0.49 18.42 86 164 300041 PRO A, 300088 SER A 4.74 225.42 -1.2 -0.53 1.63 87 165 300091 SER A, 300041 PRO A, 300088 SER A 4.62 225.89 -1.07 -0.68 1.64 97 166 300091 SER A, 300041 PRO A, 300088 SER A, 300175 LEU A 4.41 226.64 0.15 -0.22 1.26 86 167 300041 PRO A, 300088 SER A, 300175 LEU A, 300180 TYR A 4.32 226.88 0.02 0.3 1.25 69 168 300091 SER A, 300088 SER A, 300175 LEU A, 300180 TYR A 4.25 227.08 0.23 0.08 1.27 84 169 300091 SER A, 300088 SER A, 300180 TYR A 4.13 227.3 -0.97 -0.28 1.65 94 170 300091 SER A, 300088 SER A 4.08 227.46 -0.8 -0.97 1.67 117 171 300091 SER A, 300088 SER A, 300180 TYR A 4.05 227.84 -0.97 -0.28 1.65 94 172 300041 PRO A, 300088 SER A, 300175 LEU A, 300180 TYR A 4.03 228.51 0.02 0.3 1.25 69 173 300088 SER A, 300091 SER A, 300041 PRO A, 300175 LEU A, 300180 TYR A 4.02 229.37 -0.06 0.08 1.67 69 174 300041 PRO A, 300175 LEU A, 300180 TYR A 4.02 233.31 0.3 0.72 1.11 54 175 300041 PRO A, 300175 LEU A, 300180 TYR A, 300173 ALA A 4.34 233.59 0.13 0.34 1.68 54 176 300041 PRO A, 300175 LEU A, 300180 TYR A 4.43 234.26 0.3 0.72 1.11 54 177 300041 PRO A, 300175 LEU A, 300180 TYR A, 300173 ALA A 4.29 234.99 0.13 0.34 1.68 54 178 300041 PRO A, 300180 TYR A, 300173 ALA A 3.81 237.23 -1.1 0.07 2.19 54 179 300041 PRO A, 300180 TYR A, 300173 ALA A, 300153 GLU A 3.31 239.02 -1.7 -0.23 14.12 61 180 300041 PRO A, 300173 ALA A, 300153 GLU A, 300172 PRO A 3.24 241.44 -1.78 -0.53 14.11 64 181 300041 PRO A, 300153 GLU A, 300172 PRO A, 300041 ASN B 3.43 241.96 -2.55 -0.52 14.11 74 182 300041 PRO A, 300153 GLU A, 300172 PRO A, 300041 ASN B 3.49 242.6 -2.55 -0.52 14.11 74 183 300041 PRO A, 300172 PRO A, 300041 ASN B, 300042 GLY A 3.55 243.61 -1.78 -0.44 2.48 73 184 300041 PRO A, 300172 PRO A, 300041 ASN B, 300042 GLY A 3.65 243.83 -1.78 -0.44 2.48 73 185 300041 PRO A, 300172 PRO A, 300041 ASN B, 300042 GLY A 3.68 244.1 -1.78 -0.44 2.48 73 186 300172 PRO A, 300041 ASN B, 300042 GLY A, 300165 ASP B 3.62 244.84 -2.25 -0.68 14.51 82 187 300172 PRO A, 300041 ASN B, 300042 GLY A, 300165 ASP B, 300040 THR B 3.55 245.11 -1.94 -0.69 11.94 88 188 300041 ASN B, 300042 GLY A, 300165 ASP B, 300040 THR B 3.22 247.5 -2.03 -0.85 14.53 99 189 300042 GLY A, 300165 ASP B, 300040 THR B 3.21 248.73 -1.53 -0.87 18.25 96 190 300042 GLY A, 300165 ASP B, 300085 ASN B 3.27 248.8 -2.47 -0.87 18.82 95 191 300042 GLY A, 300165 ASP B, 300040 THR B, 300085 ASN B 3.23 249.12 -2.03 -0.85 14.53 99 192 300165 ASP B, 300040 THR B, 300085 ASN B 2.9 249.63 -2.57 -0.86 18.25 99 193 300042 GLY A, 300165 ASP B, 300085 ASN B 2.67 254.89 -2.47 -0.87 18.82 95 194 300042 GLY A, 300165 ASP B, 300085 ASN B, 300103 LYS B 3.54 255.95 -2.83 -0.76 26.49 87 195 300042 GLY A, 300165 ASP B, 300103 LYS B, 300010 ILE B 3.93 256.15 -0.83 -0.11 25.68 87 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
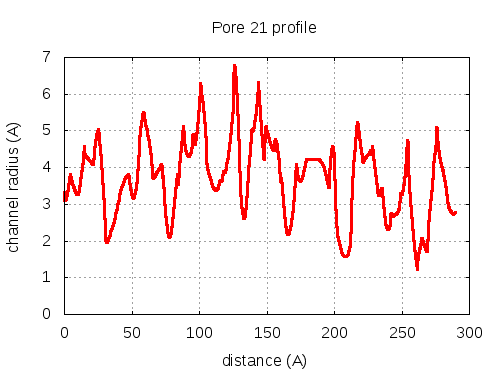
Unique lining residues set - all
100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B, 100155 VAL A, 100182 LEU A, 100171 PHE A, 100172 PRO A, 100153 GLU A, 100173 ALA A, 100172 PRO A, 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A, 100091 SER A, 100088 SER A, 100040 ARG A, 100091 SER A, 100043 HIS A, 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A, 100063 LYS A, 300020 SER B, 100003 LEU B, 100098 PHE B, 100061 ASN A, 100097 THR B, 100001 ASP B, 300072 THR B, 300070 ASP B, 100001 ASP B, 100026 SER B, 300024 ARG B, 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B, 300028 SER B, 300028 SER B, 100027 GLN B, 300027 GLN B, 100028 SER B, 100028 SER B, 27 GLN B, 28 SER B, 200027 GLN B, 200028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 300079 GLY C, 100079 GLY C, 93 ARG B, 79 GLY C, 82 TYR C, 200079 GLY C, 300082 TYR C, 200093 ARG B, 200082 TYR C, 100058 GLN C, 100080 ASP C, 200028 SER B, 28 SER B, 100027 GLN B, 200026 SER B, 100069 THR B, 100024 ARG B, 100070 ASP B, 200026 SER B, 200003 LEU B, 200001 ASP B, 100072 THR B, 200001 ASP B, 200063 LYS A, 200097 THR B, 200002 ILE B, 200098 PHE B, 200061 ASN A, 200046 GLU A, 200064 ILE A, 100020 SER B, 200063 LYS A, 100018 ARG B, 200040 ARG A, 200088 SER A, 200091 SER A, 200091 SER A, 200041 PRO A, 200088 SER A, 200175 LEU A, 200180 TYR A, 200173 ALA A, 200153 GLU A, 200172 PRO A, 200041 ASN B, 200042 GLY A, 200165 ASP B, 200040 THR B, 200085 ASN B, 200103 LYS B, 200010 ILE B
Unique lining residues set - sidechains
100156 THR A, 100170 THR A, 100041 ASN B, 100182 LEU A, 100153 GLU A, 100172 PRO A, 100041 PRO A, 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A, 100040 ARG A, 100043 HIS A, 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A, 300020 SER B, 100003 LEU B, 100061 ASN A, 100097 THR B, 100001 ASP B, 300072 THR B, 300070 ASP B, 100026 SER B, 300024 ARG B, 300069 THR B, 300028 SER B, 100027 GLN B, 300027 GLN B, 100028 SER B, 27 GLN B, 200027 GLN B, 200028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 93 ARG B, 82 TYR C, 300082 TYR C, 200093 ARG B, 200082 TYR C, 100058 GLN C, 28 SER B, 100069 THR B, 100024 ARG B, 100070 ASP B, 200026 SER B, 200003 LEU B, 100072 THR B, 200001 ASP B, 200063 LYS A, 200097 THR B, 200061 ASN A, 200046 GLU A, 200064 ILE A, 100020 SER B, 100018 ARG B, 200040 ARG A, 200091 SER A, 200041 PRO A, 200088 SER A, 200175 LEU A, 200180 TYR A, 200153 GLU A, 200172 PRO A, 200041 ASN B, 200165 ASP B, 200040 THR B, 200085 ASN B, 200103 LYS B, 200010 ILE B
Physicochemical properties of lining side-chains
Charge: 4 (13-9)
Hydropathy: -1.5
Hydrophobicity: -0.43
Polarity: 15.06
Mutability: 87
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B 3.26 0.26 -1.33 -0.78 2.52 106 2 100157 VAL A, 100170 THR A, 100041 ASN B 3.2 0.45 -1.53 -0.78 2.81 105 3 100170 THR A, 100041 ASN B, 100155 VAL A 3.2 0.61 -1.53 -0.78 2.81 105 4 100157 VAL A, 100170 THR A, 100041 ASN B 3.22 0.74 -1.53 -0.78 2.81 105 5 100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B 3.2 1.03 -1.33 -0.78 2.52 106 6 100156 THR A, 100157 VAL A, 100041 ASN B 3 1.51 -1.53 -0.78 2.81 105 7 100157 VAL A, 100170 THR A, 100041 ASN B, 100155 VAL A 2.89 2.08 -1.25 -0.79 2.95 105 8 100170 THR A, 100041 ASN B, 100155 VAL A 2.51 5.72 -1.53 -0.78 2.81 105 9 100170 THR A, 100041 ASN B, 100155 VAL A, 100182 LEU A 2.52 6.66 -0.2 -0.3 2.14 88 10 100170 THR A, 100041 ASN B, 100182 LEU A 2.78 7.02 -0.13 -0.13 1.72 88 11 100041 ASN B, 100182 LEU A, 100171 PHE A 2.76 7.2 -0.03 -0.14 2.3 79 12 100041 ASN B, 100182 LEU A, 100171 PHE A, 100172 PRO A 2.57 7.93 -0.13 -0.31 2.57 79 13 100041 ASN B, 100182 LEU A, 100171 PHE A, 100172 PRO A, 100153 GLU A 2.41 8.34 -0.8 -0.47 12.03 78 14 100041 ASN B, 100182 LEU A, 100172 PRO A, 100153 GLU A, 100173 ALA A 2.34 8.75 -0.8 -0.47 12.03 78 15 100041 ASN B, 100172 PRO A, 100153 GLU A, 100173 ALA A 2.31 9.88 -1.95 -0.88 15.01 90 16 100041 ASN B, 100153 GLU A, 100173 ALA A, 100172 PRO A 2.37 12.65 -2.25 -0.7 14.56 79 17 100041 ASN B, 100153 GLU A, 100172 PRO A 3.11 13.36 -2.87 -0.67 18.29 79 18 100041 ASN B, 100153 GLU A, 100172 PRO A, 100041 PRO A 3.36 13.89 -2.55 -0.52 14.11 74 19 100041 ASN B, 100153 GLU A, 100172 PRO A, 100041 PRO A 3.47 14.5 -2.55 -0.52 14.11 74 20 100153 GLU A, 100173 ALA A, 100172 PRO A, 100041 PRO A 3.31 16.7 -1.78 -0.53 14.11 64 21 100153 GLU A, 100173 ALA A, 100041 PRO A, 100180 TYR A 3.3 19.09 -1.7 -0.23 14.12 61 22 100173 ALA A, 100041 PRO A, 100180 TYR A 3.75 21.04 -1.1 0.07 2.19 54 23 100173 ALA A, 100041 PRO A, 100180 TYR A, 100175 LEU A 4.42 21.41 0.13 0.34 1.68 54 24 100041 PRO A, 100180 TYR A, 100175 LEU A 4.37 22.12 0.3 0.72 1.11 54 25 100173 ALA A, 100041 PRO A, 100180 TYR A, 100175 LEU A 4.46 22.49 0.13 0.34 1.68 54 26 100041 PRO A, 100180 TYR A, 100175 LEU A 4.21 27.28 0.3 0.72 1.11 54 27 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A, 100091 SER A 4.2 27.85 -0.06 0.08 1.67 69 28 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A 4.2 28.15 0.23 0.08 1.27 84 29 100180 TYR A, 100091 SER A, 100088 SER A 4.23 28.26 -0.97 -0.28 1.65 94 30 100091 SER A, 100088 SER A 4.27 28.35 -0.8 -0.97 1.67 117 31 100180 TYR A, 100091 SER A, 100088 SER A 4.33 28.52 -0.97 -0.28 1.65 94 32 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A 4.41 29 0.23 0.08 1.27 84 33 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A 4.6 29.36 0.02 0.3 1.25 69 34 100041 PRO A, 100175 LEU A, 100091 SER A, 100088 SER A 4.71 29.8 0.15 -0.22 1.26 86 35 100041 PRO A, 100091 SER A, 100088 SER A 4.82 30.76 -1.07 -0.68 1.64 97 36 100041 PRO A, 100088 SER A 5 31.9 -1.2 -0.53 1.63 87 37 100041 PRO A, 100088 SER A, 100040 ARG A 4.55 33.89 -2.3 -0.49 18.42 86 38 100041 PRO A, 100088 SER A, 100040 ARG A 3.28 36.63 -2.17 -0.44 18.99 70 39 100041 PRO A, 100088 SER A, 100040 ARG A, 100091 SER A 2.9 36.96 -1.73 -0.53 15.09 70 40 100088 SER A, 100040 ARG A, 100091 SER A 1.5 38 -1.77 -0.67 19.59 83 41 100088 SER A, 100040 ARG A 0.61 40.1 -2.45 -0.61 27.69 83 42 100040 ARG A 0.21 45.88 -4.5 -0.42 52 83 43 100040 ARG A, 100043 HIS A 1.27 46.91 -3.85 -0.08 51.8 87 44 100040 ARG A, 100046 GLU A 1.98 50.22 -4 -0.78 50.95 80 45 100040 ARG A, 100046 GLU A, 300018 ARG B 4.43 52.92 -4.17 -0.66 51.3 81 46 100040 ARG A, 100046 GLU A, 300018 ARG B, 100064 ILE A 3.53 56.18 -2 -0.04 38.51 86 47 100046 GLU A, 300018 ARG B, 100064 ILE A 3.51 56.77 -1.17 0.08 34.01 87 48 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A 3.15 59.42 -0.98 -0.14 26.35 87 49 100046 GLU A, 100064 ILE A, 100063 LYS A 3.18 60.22 0.2 -0.04 17.8 90 50 100046 GLU A, 100064 ILE A, 100063 LYS A 3.32 64.79 -0.97 0.09 33.18 84 51 100046 GLU A, 100064 ILE A, 100063 LYS A, 300020 SER B 4.97 65.29 -0.93 -0.18 25.3 92 52 100046 GLU A, 100063 LYS A, 300020 SER B, 100003 LEU B 4.84 66.16 -1.1 -0.35 25.3 80 53 100046 GLU A, 100064 ILE A, 100063 LYS A, 100003 LEU B 4.35 69.35 0.23 0.35 24.92 76 54 100046 GLU A, 100063 LYS A, 100003 LEU B, 100098 PHE B 4.23 70.36 -1 -0.3 25.73 67 55 100046 GLU A, 100063 LYS A, 100003 LEU B, 100098 PHE B, 100061 ASN A 4.16 71.06 -1.5 -0.4 21.26 76 56 100046 GLU A, 100063 LYS A, 100098 PHE B, 100061 ASN A, 100097 THR B 4.14 71.41 -2.4 -0.78 21.56 90 57 100046 GLU A, 100098 PHE B, 100061 ASN A, 100097 THR B 4.15 71.78 -2.03 -0.87 14.58 96 58 100046 GLU A, 100063 LYS A, 100098 PHE B, 100061 ASN A, 100097 THR B 4.2 72 -2.4 -0.78 21.56 90 59 100063 LYS A, 100003 LEU B, 100098 PHE B, 100061 ASN A, 100097 THR B 4 72.97 -0.94 -0.32 11.61 84 60 100063 LYS A, 100003 LEU B, 100061 ASN A, 100097 THR B 3.88 73.4 -1.08 -0.2 13.67 84 61 100063 LYS A, 100003 LEU B, 100097 THR B, 100001 ASP B 3.56 75.37 -1.08 -0.27 25.25 79 62 100063 LYS A, 100003 LEU B, 100001 ASP B 3.58 77.13 -1.2 -0.1 33.11 70 63 100063 LYS A, 100003 LEU B, 100001 ASP B, 300072 THR B 3.83 78.06 -1.08 -0.27 25.25 79 64 100063 LYS A, 100003 LEU B, 100001 ASP B, 300072 THR B, 300070 ASP B 4.1 78.27 -1.56 -0.42 30.14 81 65 100003 LEU B, 100001 ASP B, 300072 THR B, 300070 ASP B 3.84 79.15 -0.98 -0.43 25.3 83 66 100003 LEU B, 300072 THR B, 300070 ASP B, 100001 ASP B 3.8 79.32 -0.2 -0.37 13.72 82 67 100003 LEU B, 300070 ASP B, 100001 ASP B 3.09 81.29 -0.03 -0.23 17.74 70 68 100003 LEU B, 300070 ASP B 2.21 84.4 0.15 0.05 24.92 70 69 100003 LEU B, 300070 ASP B, 100026 SER B 2.09 86.85 -0.17 -0.29 17.17 85 70 100003 LEU B, 300070 ASP B, 100026 SER B, 300024 ARG B 2.4 89.03 -1.25 -0.32 25.88 85 71 300070 ASP B, 100026 SER B, 300024 ARG B 3.34 90.45 -2.93 -0.81 34.46 95 72 300070 ASP B, 300024 ARG B, 100026 SER B 3.57 91.62 -2.8 -0.75 35.03 84 73 300070 ASP B, 300024 ARG B, 100026 SER B, 300069 THR B 3.84 93.2 -2.28 -0.76 26.69 92 74 300024 ARG B, 100026 SER B, 300069 THR B 4.57 94.65 -1.87 -0.66 19.01 95 75 300024 ARG B, 100026 SER B, 300069 THR B, 300026 SER B 4.67 95.48 -1.5 -0.7 15.11 95 76 100026 SER B, 300069 THR B, 300026 SER B 4.48 98.47 -0.5 -0.79 2.81 107 77 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B 4.7 99.4 -0.48 -0.79 2.95 107 78 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B, 300028 SER B 4.86 101.19 -0.46 -0.79 3.04 107 79 100026 SER B, 300069 THR B, 300027 GLN B, 300028 SER B 5.16 101.38 -0.58 -0.84 2.52 112 80 100026 SER B, 300027 GLN B, 300028 SER B, 100027 GLN B 5.12 101.52 -1.28 -0.92 2.99 100 81 100026 SER B, 300069 THR B, 300028 SER B, 100027 GLN B 4.23 104.74 -1.35 -0.91 2.56 102 82 100026 SER B, 300028 SER B, 100027 GLN B 5.43 105.41 -1.57 -0.96 2.86 100 83 300028 SER B, 100027 GLN B, 300027 GLN B 5.72 106.03 -2.6 -1.06 2.91 95 84 300028 SER B, 100027 GLN B, 300027 GLN B, 100028 SER B 5.98 106.52 -2.05 -0.99 3.03 95 85 300028 SER B, 100027 GLN B, 100028 SER B 6.21 106.84 -1.7 -1.01 2.29 106 86 27 GLN B, 28 SER B, 200027 GLN B, 200028 SER B 6.39 107.84 -2.05 -0.99 3.03 95 87 300028 SER B, 100027 GLN B, 100028 SER B 4.99 111.36 -1.7 -1.01 2.29 106 88 300028 SER B, 100027 GLN B, 100028 SER B, 100093 ARG B 4.79 111.63 -2.4 -0.87 14.72 100 89 100027 GLN B, 100028 SER B, 100093 ARG B 4.76 112.05 -2.93 -0.83 19.07 94 90 300028 SER B, 100027 GLN B, 100028 SER B, 100093 ARG B 4.57 113.05 -2.4 -0.87 14.72 100 91 300028 SER B, 100027 GLN B, 100093 ARG B 2.5 118.44 -2.93 -0.83 19.07 94 92 300028 SER B, 100093 ARG B 2.73 119.14 -2.65 -0.7 26.84 100 93 300028 SER B, 100093 ARG B, 100082 TYR C 3.04 120.22 -2.2 -0.09 18.43 83 94 300028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B 3.85 123.27 -2.78 -0.18 26.82 83 95 300028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 300079 GLY C 6.1 124.07 -2.3 -0.3 22.13 83 96 100093 ARG B, 100082 TYR C, 300093 ARG B, 300079 GLY C, 100079 GLY C 6.53 124.71 -2.22 -0.27 22.47 72 97 93 ARG B, 79 GLY C, 82 TYR C, 200079 GLY C, 300082 TYR C 6.94 124.95 -1.58 0.04 12.4 61 98 93 ARG B, 79 GLY C, 82 TYR C, 300082 TYR C, 200093 ARG B 7.09 125.33 -2.4 0.12 22.12 66 99 300093 ARG B, 93 ARG B, 79 GLY C, 82 TYR C, 300082 TYR C 6.97 125.73 -2.4 0.12 22.12 66 100 100093 ARG B, 100082 TYR C, 300093 ARG B, 300079 GLY C, 100079 GLY C 6.66 126.41 -2.22 -0.27 22.47 72 101 100093 ARG B, 100082 TYR C, 300079 GLY C, 100079 GLY C, 200082 TYR C 5.63 128.3 -1.58 0.04 12.4 61 102 100093 ARG B, 100082 TYR C, 100079 GLY C, 200082 TYR C 5.02 129.27 -1.88 0.25 14.65 61 103 100093 ARG B, 100082 TYR C, 100079 GLY C, 100058 GLN C 3.12 131.63 -2.43 -0.3 15.13 72 104 100093 ARG B, 100079 GLY C, 100058 GLN C, 100080 ASP C 3.16 132.35 -2.2 -0.78 15.57 83 105 100093 ARG B, 100079 GLY C, 200082 TYR C, 100058 GLN C 3.47 133.45 -2.43 -0.3 15.13 72 106 100093 ARG B, 200082 TYR C, 100058 GLN C 3.65 134.43 -3.1 -0.14 19.05 72 107 100028 SER B, 100093 ARG B, 200082 TYR C, 100058 GLN C 3.57 134.68 -2.43 -0.3 15.13 72 108 100028 SER B, 100093 ARG B, 200082 TYR C, 100058 GLN C 3.47 135.14 -2.53 -0.35 14.7 83 109 100028 SER B, 100093 ARG B, 100058 GLN C 3.48 135.45 -2.93 -0.83 19.07 94 110 100028 SER B, 100093 ARG B, 200082 TYR C, 100058 GLN C 3.83 136.7 -2.53 -0.35 14.7 83 111 100028 SER B, 100093 ARG B, 200093 ARG B, 200082 TYR C 3.98 137.11 -2.78 -0.18 26.82 83 112 100028 SER B, 200093 ARG B, 200082 TYR C 3.3 139.03 -2.2 -0.09 18.43 83 113 100028 SER B, 200027 GLN B, 200093 ARG B 2.48 144.2 -2.93 -0.83 19.07 94 114 100028 SER B, 200027 GLN B, 200093 ARG B, 200028 SER B 4.08 144.73 -2.3 -0.82 15.15 94 115 100028 SER B, 200027 GLN B, 200028 SER B, 200093 ARG B 4.49 145.04 -2.4 -0.87 14.72 100 116 200027 GLN B, 200028 SER B, 200093 ARG B 4.83 145.53 -2.93 -0.83 19.07 94 117 100028 SER B, 200027 GLN B, 200028 SER B, 200093 ARG B 5.08 146.2 -2.4 -0.87 14.72 100 118 100028 SER B, 200027 GLN B, 200028 SER B 5.07 149.7 -1.7 -1.01 2.29 106 119 300028 SER B, 300027 GLN B, 27 GLN B, 28 SER B 5.99 150.68 -2.05 -0.99 3.03 95 120 100027 GLN B, 100028 SER B, 200027 GLN B, 200028 SER B 5.49 151.9 -2.05 -0.99 3.03 95 121 100028 SER B, 200027 GLN B, 100027 GLN B 5.22 152.57 -1.57 -0.96 2.86 100 122 100028 SER B, 200027 GLN B, 200026 SER B 4.97 153.19 -1.57 -0.96 2.86 100 123 100028 SER B, 200027 GLN B, 200026 SER B, 100069 THR B 4.51 155.54 -1.35 -0.91 2.56 102 124 100028 SER B, 200027 GLN B, 100027 GLN B, 200026 SER B 4.82 155.94 -1.28 -0.92 2.99 100 125 100026 SER B, 100028 SER B, 100027 GLN B, 200026 SER B, 100069 THR B 4.92 158.97 -0.46 -0.79 3.04 107 126 100026 SER B, 100027 GLN B, 200026 SER B, 100069 THR B 5.01 159.9 -0.48 -0.79 2.95 107 127 100026 SER B, 200026 SER B, 100069 THR B 4.97 161.67 -0.5 -0.79 2.81 107 128 100026 SER B, 200026 SER B, 100069 THR B, 100024 ARG B 4.68 162.93 -1.5 -0.7 15.11 95 129 200026 SER B, 100069 THR B, 100024 ARG B 4.34 164.65 -1.87 -0.66 19.01 95 130 200026 SER B, 100069 THR B, 100024 ARG B, 100070 ASP B 3.91 165.83 -2.28 -0.76 26.69 92 131 200026 SER B, 100024 ARG B, 100070 ASP B 3.63 166.55 -2.8 -0.75 35.03 84 132 100024 ARG B, 100070 ASP B, 200026 SER B 3.45 168.59 -2.93 -0.81 34.46 95 133 100024 ARG B, 100070 ASP B, 200026 SER B, 200003 LEU B 2.76 170.37 -1.25 -0.32 25.88 85 134 100070 ASP B, 200026 SER B, 200003 LEU B 2.09 173.36 -0.17 -0.29 17.17 85 135 100070 ASP B, 200003 LEU B 2.18 175.9 0.15 0.05 24.92 70 136 100070 ASP B, 200003 LEU B, 200001 ASP B 2.96 177.9 -0.03 -0.23 17.74 70 137 100070 ASP B, 200003 LEU B, 200001 ASP B, 100072 THR B 3.93 178.02 -0.2 -0.37 13.72 82 138 100070 ASP B, 200003 LEU B, 100072 THR B, 200001 ASP B 3.95 179.03 -0.98 -0.43 25.3 83 139 100070 ASP B, 200003 LEU B, 100072 THR B, 200001 ASP B, 200063 LYS A 4.09 179.27 -1.56 -0.42 30.14 81 140 200003 LEU B, 100072 THR B, 200001 ASP B, 200063 LYS A 4.04 180.1 -1.08 -0.27 25.25 79 141 200003 LEU B, 200001 ASP B, 200063 LYS A 3.72 182.44 -1.2 -0.1 33.11 70 142 200003 LEU B, 200001 ASP B, 200063 LYS A, 200097 THR B 3.51 183.8 -1.08 -0.27 25.25 79 143 200003 LEU B, 200063 LYS A, 200097 THR B, 200002 ILE B 3.49 184.21 -0.3 -0.21 13.67 77 144 200003 LEU B, 200063 LYS A, 200097 THR B, 200098 PHE B 3.49 184.58 -0.3 -0.21 13.67 77 145 200003 LEU B, 200063 LYS A, 200097 THR B, 200098 PHE B, 200061 ASN A 3.53 185.3 -0.94 -0.32 11.61 84 146 200063 LYS A, 200097 THR B, 200098 PHE B, 200061 ASN A, 200046 GLU A 3.83 185.46 -2.4 -0.78 21.56 90 147 200097 THR B, 200098 PHE B, 200061 ASN A, 200046 GLU A 4.01 185.69 -2.03 -0.87 14.58 96 148 200063 LYS A, 200097 THR B, 200098 PHE B, 200061 ASN A, 200046 GLU A 4.44 186.2 -2.4 -0.78 21.56 90 149 200003 LEU B, 200063 LYS A, 200098 PHE B, 200061 ASN A, 200046 GLU A 4.66 187.06 -1.5 -0.4 21.26 76 150 200003 LEU B, 200063 LYS A, 200098 PHE B, 200046 GLU A 4.84 188.16 -1 -0.3 25.73 67 151 200003 LEU B, 200063 LYS A, 200046 GLU A, 200064 ILE A 4.83 191.54 0.23 0.35 24.92 76 152 200003 LEU B, 200063 LYS A, 200046 GLU A, 100020 SER B 4.61 191.89 -1.1 -0.35 25.3 80 153 200063 LYS A, 200046 GLU A, 200064 ILE A, 100020 SER B 4.36 192.52 -0.93 -0.18 25.3 92 154 200063 LYS A, 200046 GLU A, 200064 ILE A 3.54 197.14 -0.97 0.09 33.18 84 155 200046 GLU A, 200064 ILE A, 200063 LYS A 3.75 197.93 0.2 -0.04 17.8 90 156 200046 GLU A, 200064 ILE A, 200063 LYS A, 100018 ARG B 3.85 201.07 -0.98 -0.14 26.35 87 157 200046 GLU A, 200064 ILE A, 100018 ARG B, 200040 ARG A 3.53 204.28 -2 -0.04 38.51 86 158 200046 GLU A, 100018 ARG B, 200040 ARG A 3.93 207 -4.17 -0.66 51.3 81 159 200046 GLU A, 200064 ILE A, 200040 ARG A 4.08 207.52 -1.17 0.08 34.01 87 160 200046 GLU A, 200040 ARG A 1.96 211 -4 -0.78 50.95 80 161 200040 ARG A -0.05 217.1 -4.5 -0.42 52 83 162 200040 ARG A, 200088 SER A 0.06 217.87 -2.45 -0.61 27.69 83 163 200040 ARG A, 200088 SER A, 200091 SER A 0.38 218.29 -1.77 -0.67 19.59 83 164 200040 ARG A, 200088 SER A, 200091 SER A 0.88 218.52 -1.9 -0.73 19.02 100 165 200040 ARG A, 200088 SER A, 200091 SER A, 200041 PRO A 1.48 218.78 -1.83 -0.57 14.66 86 166 200040 ARG A, 200088 SER A, 200091 SER A, 200041 PRO A 2.13 219.52 -1.73 -0.53 15.09 70 167 200040 ARG A, 200088 SER A, 200041 PRO A 3.36 221.99 -2.17 -0.44 18.99 70 168 200040 ARG A, 200041 PRO A, 200088 SER A 4.91 224.13 -2.3 -0.49 18.42 86 169 200041 PRO A, 200088 SER A 4.72 225.5 -1.2 -0.53 1.63 87 170 200091 SER A, 200041 PRO A, 200088 SER A 4.6 225.97 -1.07 -0.68 1.64 97 171 200091 SER A, 200041 PRO A, 200088 SER A, 200175 LEU A 4.4 226.68 0.15 -0.22 1.26 86 172 200041 PRO A, 200088 SER A, 200175 LEU A, 200180 TYR A 4.31 226.92 0.02 0.3 1.25 69 173 200091 SER A, 200088 SER A, 200175 LEU A, 200180 TYR A 4.24 227.11 0.23 0.08 1.27 84 174 200091 SER A, 200088 SER A, 200180 TYR A 4.12 227.32 -0.97 -0.28 1.65 94 175 200091 SER A, 200088 SER A 4.08 227.5 -0.8 -0.97 1.67 117 176 200091 SER A, 200088 SER A, 200180 TYR A 4.04 227.93 -0.97 -0.28 1.65 94 177 200041 PRO A, 200088 SER A, 200175 LEU A, 200180 TYR A 4.03 228.62 0.02 0.3 1.25 69 178 200088 SER A, 200091 SER A, 200041 PRO A, 200175 LEU A, 200180 TYR A 4.02 229.51 -0.06 0.08 1.67 69 179 200041 PRO A, 200175 LEU A, 200180 TYR A 4.03 233.18 0.3 0.72 1.11 54 180 200041 PRO A, 200175 LEU A, 200180 TYR A, 200173 ALA A 4.28 233.62 0.13 0.34 1.68 54 181 200041 PRO A, 200175 LEU A, 200180 TYR A 4.44 234.19 0.3 0.72 1.11 54 182 200041 PRO A, 200175 LEU A, 200180 TYR A, 200173 ALA A 4.29 235.04 0.13 0.34 1.68 54 183 200041 PRO A, 200180 TYR A, 200173 ALA A 3.79 237.29 -1.1 0.07 2.19 54 184 200041 PRO A, 200180 TYR A, 200173 ALA A, 200153 GLU A 3.31 239.06 -1.7 -0.23 14.12 61 185 200041 PRO A, 200173 ALA A, 200153 GLU A, 200172 PRO A 3.24 241.46 -1.78 -0.53 14.11 64 186 200041 PRO A, 200153 GLU A, 200172 PRO A, 200041 ASN B 3.43 241.98 -2.55 -0.52 14.11 74 187 200041 PRO A, 200153 GLU A, 200172 PRO A, 200041 ASN B 3.49 242.64 -2.55 -0.52 14.11 74 188 200041 PRO A, 200172 PRO A, 200041 ASN B, 200042 GLY A 3.55 243.64 -1.78 -0.44 2.48 73 189 200041 PRO A, 200172 PRO A, 200041 ASN B, 200042 GLY A 3.65 243.85 -1.78 -0.44 2.48 73 190 200041 PRO A, 200172 PRO A, 200041 ASN B, 200042 GLY A 3.68 244.11 -1.78 -0.44 2.48 73 191 200172 PRO A, 200041 ASN B, 200042 GLY A, 200165 ASP B 3.61 244.86 -2.25 -0.68 14.51 82 192 200172 PRO A, 200041 ASN B, 200042 GLY A, 200165 ASP B, 200040 THR B 3.54 245.14 -1.94 -0.69 11.94 88 193 200041 ASN B, 200042 GLY A, 200165 ASP B, 200040 THR B 3.22 247.53 -2.03 -0.85 14.53 99 194 200042 GLY A, 200165 ASP B, 200040 THR B 3.21 248.74 -1.53 -0.87 18.25 96 195 200042 GLY A, 200165 ASP B, 200085 ASN B 3.27 248.81 -2.47 -0.87 18.82 95 196 200042 GLY A, 200165 ASP B, 200040 THR B, 200085 ASN B 3.23 249.13 -2.03 -0.85 14.53 99 197 200165 ASP B, 200040 THR B, 200085 ASN B 2.89 249.65 -2.57 -0.86 18.25 99 198 200042 GLY A, 200165 ASP B, 200085 ASN B 2.67 254.9 -2.47 -0.87 18.82 95 199 200042 GLY A, 200165 ASP B, 200085 ASN B, 200103 LYS B 3.54 255.95 -2.83 -0.76 26.49 87 200 200042 GLY A, 200165 ASP B, 200103 LYS B, 200010 ILE B 3.93 256.16 -0.83 -0.11 25.68 87 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
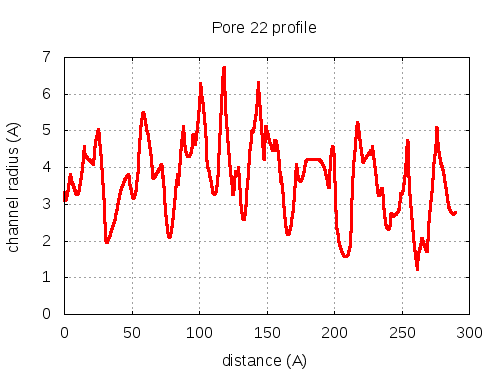
Unique lining residues set - all
100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B, 100155 VAL A, 100182 LEU A, 100171 PHE A, 100172 PRO A, 100153 GLU A, 100173 ALA A, 100172 PRO A, 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A, 100091 SER A, 100088 SER A, 100040 ARG A, 100091 SER A, 100043 HIS A, 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A, 100063 LYS A, 300020 SER B, 100003 LEU B, 100098 PHE B, 100061 ASN A, 100097 THR B, 100002 ILE B, 100001 ASP B, 300072 THR B, 300070 ASP B, 100001 ASP B, 100026 SER B, 300024 ARG B, 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B, 300028 SER B, 100027 GLN B, 300028 SER B, 100028 SER B, 300027 GLN B, 27 GLN B, 28 SER B, 200027 GLN B, 200028 SER B, 100028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 300079 GLY C, 100079 GLY C, 93 ARG B, 79 GLY C, 82 TYR C, 200079 GLY C, 300082 TYR C, 200093 ARG B, 200082 TYR C, 100058 GLN C, 100080 ASP C, 200028 SER B, 28 SER B, 200026 SER B, 100069 THR B, 100027 GLN B, 100024 ARG B, 100070 ASP B, 200026 SER B, 200003 LEU B, 200001 ASP B, 100072 THR B, 200001 ASP B, 200063 LYS A, 200097 THR B, 200061 ASN A, 200098 PHE B, 200046 GLU A, 200064 ILE A, 100020 SER B, 200063 LYS A, 100018 ARG B, 200040 ARG A, 200088 SER A, 200091 SER A, 200091 SER A, 200041 PRO A, 200088 SER A, 200175 LEU A, 200180 TYR A, 200173 ALA A, 200153 GLU A, 200172 PRO A, 200041 ASN B, 200172 PRO A, 200171 PHE A, 200182 LEU A, 200170 THR A, 200155 VAL A, 200156 THR A, 200157 VAL A, 200040 THR B
Unique lining residues set - sidechains
100156 THR A, 100170 THR A, 100041 ASN B, 100182 LEU A, 100153 GLU A, 100172 PRO A, 100041 PRO A, 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A, 100040 ARG A, 100043 HIS A, 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A, 300020 SER B, 100003 LEU B, 100061 ASN A, 100097 THR B, 100001 ASP B, 300072 THR B, 300070 ASP B, 100026 SER B, 300024 ARG B, 300069 THR B, 100027 GLN B, 300028 SER B, 300027 GLN B, 27 GLN B, 200027 GLN B, 200028 SER B, 100028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 93 ARG B, 82 TYR C, 300082 TYR C, 200093 ARG B, 200082 TYR C, 100058 GLN C, 28 SER B, 100069 THR B, 100024 ARG B, 100070 ASP B, 200026 SER B, 200003 LEU B, 100072 THR B, 200001 ASP B, 200063 LYS A, 200097 THR B, 200061 ASN A, 200046 GLU A, 200064 ILE A, 100020 SER B, 100018 ARG B, 200040 ARG A, 200091 SER A, 200041 PRO A, 200088 SER A, 200175 LEU A, 200180 TYR A, 200153 GLU A, 200172 PRO A, 200041 ASN B, 200182 LEU A, 200170 THR A, 200156 THR A,
Physicochemical properties of lining side-chains
Charge: 4 (12-8)
Hydropathy: -1.5
Hydrophobicity: -0.44
Polarity: 14.39
Mutability: 86
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B 3.26 0.27 -1.33 -0.78 2.52 106 2 100157 VAL A, 100170 THR A, 100041 ASN B 3.2 0.45 -1.53 -0.78 2.81 105 3 100170 THR A, 100041 ASN B, 100155 VAL A 3.19 0.62 -1.53 -0.78 2.81 105 4 100157 VAL A, 100170 THR A, 100041 ASN B 3.2 0.77 -1.53 -0.78 2.81 105 5 100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B 3.19 1.05 -1.33 -0.78 2.52 106 6 100156 THR A, 100157 VAL A, 100041 ASN B 3.04 1.59 -1.53 -0.78 2.81 105 7 100170 THR A, 100041 ASN B, 100155 VAL A 2.62 5.8 -1.53 -0.78 2.81 105 8 100170 THR A, 100041 ASN B, 100155 VAL A, 100182 LEU A 2.61 6.54 -0.2 -0.3 2.14 88 9 100170 THR A, 100041 ASN B, 100182 LEU A 2.8 6.87 -0.13 -0.13 1.72 88 10 100041 ASN B, 100182 LEU A, 100171 PHE A 2.76 7.23 -0.03 -0.14 2.3 79 11 100041 ASN B, 100182 LEU A, 100171 PHE A, 100172 PRO A 2.57 7.96 -0.13 -0.31 2.57 79 12 100041 ASN B, 100182 LEU A, 100171 PHE A, 100172 PRO A, 100153 GLU A 2.4 8.49 -0.8 -0.47 12.03 78 13 100041 ASN B, 100182 LEU A, 100172 PRO A, 100153 GLU A, 100173 ALA A 2.32 8.97 -0.8 -0.47 12.03 78 14 100041 ASN B, 100172 PRO A, 100153 GLU A, 100173 ALA A 2.26 9.54 -1.95 -0.88 15.01 90 15 100041 ASN B, 100153 GLU A, 100173 ALA A, 100172 PRO A 2.24 12.84 -2.25 -0.7 14.56 79 16 100041 ASN B, 100153 GLU A, 100172 PRO A 3.13 13.2 -2.87 -0.67 18.29 79 17 100041 ASN B, 100153 GLU A, 100172 PRO A, 100041 PRO A 3.33 13.76 -2.55 -0.52 14.11 74 18 100041 ASN B, 100153 GLU A, 100172 PRO A, 100041 PRO A 3.6 14.67 -2.55 -0.52 14.11 74 19 100153 GLU A, 100173 ALA A, 100172 PRO A, 100041 PRO A 3.4 16.96 -1.78 -0.53 14.11 64 20 100153 GLU A, 100173 ALA A, 100041 PRO A, 100180 TYR A 3.35 18.88 -1.7 -0.23 14.12 61 21 100173 ALA A, 100041 PRO A, 100180 TYR A 3.49 20.95 -1.1 0.07 2.19 54 22 100173 ALA A, 100041 PRO A, 100180 TYR A, 100175 LEU A 4.09 21.36 0.13 0.34 1.68 54 23 100041 PRO A, 100180 TYR A, 100175 LEU A 4.38 22.07 0.3 0.72 1.11 54 24 100173 ALA A, 100041 PRO A, 100180 TYR A, 100175 LEU A 4.4 22.43 0.13 0.34 1.68 54 25 100041 PRO A, 100180 TYR A, 100175 LEU A 4.3 27.21 0.3 0.72 1.11 54 26 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A, 100091 SER A 4.26 27.8 -0.06 0.08 1.67 69 27 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A 4.22 28.12 0.23 0.08 1.27 84 28 100180 TYR A, 100091 SER A, 100088 SER A 4.18 28.23 -0.97 -0.28 1.65 94 29 100091 SER A, 100088 SER A 4.17 28.32 -0.8 -0.97 1.67 117 30 100180 TYR A, 100091 SER A, 100088 SER A 4.17 28.49 -0.97 -0.28 1.65 94 31 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A 4.2 28.97 0.23 0.08 1.27 84 32 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A 4.32 29.34 0.02 0.3 1.25 69 33 100041 PRO A, 100175 LEU A, 100091 SER A, 100088 SER A 4.44 29.78 0.15 -0.22 1.26 86 34 100041 PRO A, 100091 SER A, 100088 SER A 4.58 30.75 -1.07 -0.68 1.64 97 35 100041 PRO A, 100088 SER A 4.94 31.89 -1.2 -0.53 1.63 87 36 100041 PRO A, 100088 SER A, 100040 ARG A 4.51 33.93 -2.3 -0.49 18.42 86 37 100041 PRO A, 100088 SER A, 100040 ARG A 3 36.29 -2.17 -0.44 18.99 70 38 100041 PRO A, 100088 SER A, 100040 ARG A, 100091 SER A 2.38 36.98 -1.73 -0.53 15.09 70 39 100088 SER A, 100040 ARG A, 100091 SER A 1.85 38.11 -1.77 -0.67 19.59 83 40 100088 SER A, 100040 ARG A 1.81 39.07 -2.45 -0.61 27.69 83 41 100040 ARG A 1.85 44.91 -4.5 -0.42 52 83 42 100040 ARG A, 100043 HIS A 2.59 46.14 -3.85 -0.08 51.8 87 43 100040 ARG A, 100046 GLU A 2.83 50.39 -4 -0.78 50.95 80 44 100040 ARG A, 100046 GLU A, 300018 ARG B 3.68 52.52 -4.17 -0.66 51.3 81 45 100040 ARG A, 100046 GLU A, 300018 ARG B, 100064 ILE A 3.55 56.4 -2 -0.04 38.51 86 46 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A 3.66 59.77 -0.98 -0.14 26.35 87 47 100046 GLU A, 100064 ILE A, 100063 LYS A 3.53 60.56 0.2 -0.04 17.8 90 48 100046 GLU A, 100064 ILE A, 100063 LYS A 3.32 65.06 -0.97 0.09 33.18 84 49 100046 GLU A, 100064 ILE A, 100063 LYS A, 300020 SER B 4.51 65.41 -0.93 -0.18 25.3 92 50 100046 GLU A, 100063 LYS A, 300020 SER B, 100003 LEU B 4.82 65.84 -1.1 -0.35 25.3 80 51 100046 GLU A, 100064 ILE A, 100063 LYS A, 100003 LEU B 5.06 69.97 0.23 0.35 24.92 76 52 100046 GLU A, 100063 LYS A, 100003 LEU B, 100098 PHE B 4.93 70.81 -1 -0.3 25.73 67 53 100046 GLU A, 100063 LYS A, 100003 LEU B, 100098 PHE B, 100061 ASN A 4.69 71.29 -1.5 -0.4 21.26 76 54 100046 GLU A, 100063 LYS A, 100098 PHE B, 100061 ASN A, 100097 THR B 4.43 71.46 -2.4 -0.78 21.56 90 55 100046 GLU A, 100098 PHE B, 100061 ASN A, 100097 THR B 3.94 71.67 -2.03 -0.87 14.58 96 56 100046 GLU A, 100063 LYS A, 100098 PHE B, 100061 ASN A, 100097 THR B 3.75 71.89 -2.4 -0.78 21.56 90 57 100063 LYS A, 100003 LEU B, 100098 PHE B, 100061 ASN A, 100097 THR B 3.48 72.8 -0.94 -0.32 11.61 84 58 100063 LYS A, 100003 LEU B, 100098 PHE B, 100097 THR B 3.46 73.23 -0.3 -0.21 13.67 77 59 100063 LYS A, 100003 LEU B, 100097 THR B, 100002 ILE B 3.48 73.67 -0.3 -0.21 13.67 77 60 100063 LYS A, 100003 LEU B, 100097 THR B, 100001 ASP B 3.52 75.18 -1.08 -0.27 25.25 79 61 100063 LYS A, 100003 LEU B, 100001 ASP B 3.77 76.98 -1.2 -0.1 33.11 70 62 100063 LYS A, 100003 LEU B, 100001 ASP B, 300072 THR B 4.02 77.98 -1.08 -0.27 25.25 79 63 100063 LYS A, 100003 LEU B, 100001 ASP B, 300072 THR B, 300070 ASP B 4.12 78.19 -1.56 -0.42 30.14 81 64 100003 LEU B, 100001 ASP B, 300072 THR B, 300070 ASP B 3.9 79.1 -0.98 -0.43 25.3 83 65 100003 LEU B, 300072 THR B, 300070 ASP B, 100001 ASP B 3.88 79.26 -0.2 -0.37 13.72 82 66 100003 LEU B, 300070 ASP B, 100001 ASP B 2.99 81.27 -0.03 -0.23 17.74 70 67 100003 LEU B, 300070 ASP B 2.24 84.4 0.15 0.05 24.92 70 68 100003 LEU B, 300070 ASP B, 100026 SER B 2.18 86.87 -0.17 -0.29 17.17 85 69 100003 LEU B, 300070 ASP B, 100026 SER B, 300024 ARG B 2.57 88.63 -1.25 -0.32 25.88 85 70 300070 ASP B, 100026 SER B, 300024 ARG B 3.27 90.42 -2.93 -0.81 34.46 95 71 300070 ASP B, 300024 ARG B, 100026 SER B 3.57 91.33 -2.8 -0.75 35.03 84 72 300070 ASP B, 300024 ARG B, 100026 SER B, 300069 THR B 3.78 93.35 -2.28 -0.76 26.69 92 73 300024 ARG B, 100026 SER B, 300069 THR B 4.66 94.39 -1.87 -0.66 19.01 95 74 300024 ARG B, 100026 SER B, 300069 THR B, 300026 SER B 4.6 95.6 -1.5 -0.7 15.11 95 75 100026 SER B, 300069 THR B, 300026 SER B 4.51 98.85 -0.5 -0.79 2.81 107 76 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B, 300028 SER B 4.83 101.26 -0.46 -0.79 3.04 107 77 100026 SER B, 300027 GLN B, 100027 GLN B, 300028 SER B 5.05 101.57 -1.28 -0.92 2.99 100 78 100026 SER B, 300069 THR B, 100027 GLN B, 300028 SER B 4.22 104.42 -1.35 -0.91 2.56 102 79 100026 SER B, 100027 GLN B, 300028 SER B 5.35 105.1 -1.57 -0.96 2.86 100 80 300027 GLN B, 100027 GLN B, 300028 SER B 5.66 105.75 -1.57 -0.96 2.86 100 81 100027 GLN B, 300028 SER B, 100028 SER B, 300027 GLN B 5.95 106.7 -2.05 -0.99 3.03 95 82 27 GLN B, 28 SER B, 200027 GLN B, 200028 SER B 6.42 107.65 -2.05 -0.99 3.03 95 83 100027 GLN B, 300028 SER B, 100028 SER B 5.2 111.25 -1.7 -1.01 2.29 106 84 100027 GLN B, 300028 SER B, 100028 SER B, 100093 ARG B 4.76 111.57 -2.4 -0.87 14.72 100 85 100027 GLN B, 100028 SER B, 100093 ARG B 4.45 111.95 -2.93 -0.83 19.07 94 86 100027 GLN B, 300028 SER B, 100028 SER B, 100093 ARG B 4.27 112.87 -2.4 -0.87 14.72 100 87 100027 GLN B, 300028 SER B, 100028 SER B, 100093 ARG B 4.14 113.57 -2.3 -0.82 15.15 94 88 100027 GLN B, 300028 SER B, 100093 ARG B 2.77 118.3 -2.93 -0.83 19.07 94 89 300028 SER B, 100093 ARG B 2.84 119.02 -2.65 -0.7 26.84 100 90 300028 SER B, 100093 ARG B, 100082 TYR C 3.03 120.14 -2.2 -0.09 18.43 83 91 300028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B 3.68 123.21 -2.78 -0.18 26.82 83 92 300028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 300079 GLY C 6.24 124.03 -2.3 -0.3 22.13 83 93 100093 ARG B, 100082 TYR C, 300093 ARG B, 300079 GLY C, 100079 GLY C 6.67 124.67 -2.22 -0.27 22.47 72 94 93 ARG B, 79 GLY C, 82 TYR C, 200079 GLY C, 300082 TYR C 6.94 124.91 -1.58 0.04 12.4 61 95 93 ARG B, 79 GLY C, 82 TYR C, 300082 TYR C, 200093 ARG B 6.96 125.3 -2.4 0.12 22.12 66 96 300093 ARG B, 93 ARG B, 79 GLY C, 82 TYR C, 300082 TYR C 6.83 125.71 -2.4 0.12 22.12 66 97 100093 ARG B, 100082 TYR C, 300093 ARG B, 300079 GLY C, 100079 GLY C 6.61 126.41 -2.22 -0.27 22.47 72 98 100093 ARG B, 100082 TYR C, 300079 GLY C, 100079 GLY C, 200082 TYR C 5.89 128.32 -1.58 0.04 12.4 61 99 100093 ARG B, 100082 TYR C, 100079 GLY C, 100058 GLN C 3.21 131.64 -2.43 -0.3 15.13 72 100 100093 ARG B, 100079 GLY C, 100058 GLN C, 100080 ASP C 3.06 132.35 -2.2 -0.78 15.57 83 101 100093 ARG B, 100079 GLY C, 200082 TYR C, 100058 GLN C 3.14 133.45 -2.43 -0.3 15.13 72 102 100093 ARG B, 200082 TYR C, 100058 GLN C 3.87 134.45 -3.1 -0.14 19.05 72 103 100028 SER B, 100093 ARG B, 200082 TYR C, 100058 GLN C 3.79 135.03 -2.53 -0.35 14.7 83 104 100028 SER B, 100093 ARG B, 100058 GLN C 3.79 135.31 -2.93 -0.83 19.07 94 105 100028 SER B, 100093 ARG B, 200082 TYR C, 100058 GLN C 3.65 136.41 -2.53 -0.35 14.7 83 106 100028 SER B, 100093 ARG B, 200082 TYR C 3.58 136.79 -2.2 -0.09 18.43 83 107 100028 SER B, 100093 ARG B, 200093 ARG B, 200082 TYR C 3.5 137.24 -2.78 -0.18 26.82 83 108 100028 SER B, 200093 ARG B, 200082 TYR C 3.28 139.27 -2.2 -0.09 18.43 83 109 200027 GLN B, 100028 SER B, 200093 ARG B 3.16 144.42 -2.93 -0.83 19.07 94 110 200027 GLN B, 100028 SER B, 200093 ARG B, 200028 SER B 3.77 144.86 -2.3 -0.82 15.15 94 111 200027 GLN B, 200028 SER B, 100028 SER B, 200093 ARG B 3.99 145.08 -2.4 -0.87 14.72 100 112 200027 GLN B, 200028 SER B, 200093 ARG B 4.22 145.43 -2.93 -0.83 19.07 94 113 200027 GLN B, 200028 SER B, 100028 SER B, 200093 ARG B 4.94 145.92 -2.4 -0.87 14.72 100 114 200027 GLN B, 200028 SER B, 100028 SER B 5.36 149.53 -1.7 -1.01 2.29 106 115 300028 SER B, 300027 GLN B, 27 GLN B, 28 SER B 6.43 150.46 -2.05 -0.99 3.03 95 116 100027 GLN B, 200027 GLN B, 100028 SER B, 200028 SER B 6.24 151.61 -2.05 -0.99 3.03 95 117 100027 GLN B, 200027 GLN B, 100028 SER B 5.9 152.29 -2.6 -1.06 2.91 95 118 200027 GLN B, 100028 SER B, 200026 SER B 5.51 152.94 -1.57 -0.96 2.86 100 119 200027 GLN B, 100028 SER B, 200026 SER B, 100069 THR B 4.14 155.6 -1.35 -0.91 2.56 102 120 200027 GLN B, 100028 SER B, 200026 SER B, 100027 GLN B 5.1 155.81 -1.28 -0.92 2.99 100 121 100026 SER B, 100028 SER B, 200026 SER B, 100069 THR B, 100027 GLN B 4.51 158.74 -0.46 -0.79 3.04 107 122 100026 SER B, 200026 SER B, 100069 THR B, 100027 GLN B 4.38 159.68 -0.48 -0.79 2.95 107 123 100026 SER B, 200026 SER B, 100069 THR B 4.32 161.57 -0.5 -0.79 2.81 107 124 100026 SER B, 200026 SER B, 100069 THR B, 100024 ARG B 4.64 162.84 -1.5 -0.7 15.11 95 125 200026 SER B, 100069 THR B, 100024 ARG B 4.39 164.56 -1.87 -0.66 19.01 95 126 200026 SER B, 100069 THR B, 100024 ARG B, 100070 ASP B 3.71 165.77 -2.28 -0.76 26.69 92 127 200026 SER B, 100024 ARG B, 100070 ASP B 3.59 166.51 -2.8 -0.75 35.03 84 128 100024 ARG B, 100070 ASP B, 200026 SER B 3.22 168.58 -2.93 -0.81 34.46 95 129 100024 ARG B, 100070 ASP B, 200026 SER B, 200003 LEU B 2.46 170.38 -1.25 -0.32 25.88 85 130 100070 ASP B, 200026 SER B, 200003 LEU B 2.09 173.4 -0.17 -0.29 17.17 85 131 100070 ASP B, 200003 LEU B 2.29 175.95 0.15 0.05 24.92 70 132 100070 ASP B, 200003 LEU B, 200001 ASP B 3.03 177.91 -0.03 -0.23 17.74 70 133 100070 ASP B, 200003 LEU B, 100072 THR B, 200001 ASP B 3.83 178.86 -0.98 -0.43 25.3 83 134 100070 ASP B, 200003 LEU B, 100072 THR B, 200001 ASP B, 200063 LYS A 4.19 179.08 -1.56 -0.42 30.14 81 135 200003 LEU B, 100072 THR B, 200001 ASP B, 200063 LYS A 3.99 180.32 -1.08 -0.27 25.25 79 136 200003 LEU B, 200001 ASP B, 200063 LYS A 3.67 182.13 -1.2 -0.1 33.11 70 137 200003 LEU B, 200001 ASP B, 200063 LYS A, 200097 THR B 3.53 183.98 -1.08 -0.27 25.25 79 138 200003 LEU B, 200063 LYS A, 200097 THR B, 200061 ASN A 3.63 184.38 -1.08 -0.2 13.67 84 139 200003 LEU B, 200063 LYS A, 200097 THR B, 200061 ASN A, 200098 PHE B 3.72 185.17 -0.94 -0.32 11.61 84 140 200063 LYS A, 200097 THR B, 200061 ASN A, 200098 PHE B, 200046 GLU A 4.07 185.36 -2.4 -0.78 21.56 90 141 200097 THR B, 200061 ASN A, 200098 PHE B, 200046 GLU A 4.19 185.93 -2.03 -0.87 14.58 96 142 200063 LYS A, 200097 THR B, 200061 ASN A, 200098 PHE B, 200046 GLU A 4.48 186.67 -2.4 -0.78 21.56 90 143 200003 LEU B, 200063 LYS A, 200061 ASN A, 200098 PHE B, 200046 GLU A 4.54 187.7 -1.5 -0.4 21.26 76 144 200003 LEU B, 200063 LYS A, 200098 PHE B, 200046 GLU A 4.57 188.86 -1 -0.3 25.73 67 145 200003 LEU B, 200063 LYS A, 200046 GLU A, 200064 ILE A 4.47 191.42 0.23 0.35 24.92 76 146 200003 LEU B, 200063 LYS A, 200046 GLU A, 100020 SER B 4.29 192.26 -1.1 -0.35 25.3 80 147 200063 LYS A, 200046 GLU A, 200064 ILE A, 100020 SER B 4.19 193.02 -0.93 -0.18 25.3 92 148 200063 LYS A, 200046 GLU A, 200064 ILE A 3.81 197.71 -0.97 0.09 33.18 84 149 200046 GLU A, 200064 ILE A, 200063 LYS A, 100018 ARG B 3.74 200.93 -0.98 -0.14 26.35 87 150 200046 GLU A, 200064 ILE A, 100018 ARG B, 200040 ARG A 3.58 204.2 -2 -0.04 38.51 86 151 200046 GLU A, 100018 ARG B, 200040 ARG A 3.91 206.96 -4.17 -0.66 51.3 81 152 200046 GLU A, 200064 ILE A, 200040 ARG A 4.12 207.47 -1.17 0.08 34.01 87 153 200046 GLU A, 200040 ARG A 2.19 210.96 -4 -0.78 50.95 80 154 200040 ARG A 0.21 217.1 -4.5 -0.42 52 83 155 200040 ARG A, 200088 SER A 0.25 217.87 -2.45 -0.61 27.69 83 156 200040 ARG A, 200088 SER A, 200091 SER A 0.5 218.28 -1.77 -0.67 19.59 83 157 200040 ARG A, 200088 SER A, 200091 SER A 0.92 218.51 -1.9 -0.73 19.02 100 158 200040 ARG A, 200088 SER A, 200091 SER A, 200041 PRO A 1.45 218.78 -1.83 -0.57 14.66 86 159 200040 ARG A, 200088 SER A, 200091 SER A, 200041 PRO A 2.05 219.53 -1.73 -0.53 15.09 70 160 200040 ARG A, 200088 SER A, 200041 PRO A 3.24 222.04 -2.17 -0.44 18.99 70 161 200040 ARG A, 200041 PRO A, 200088 SER A 4.9 224.17 -2.3 -0.49 18.42 86 162 200041 PRO A, 200088 SER A 4.86 225.09 -1.2 -0.53 1.63 87 163 200091 SER A, 200041 PRO A, 200088 SER A 4.55 226.03 -1.07 -0.68 1.64 97 164 200091 SER A, 200041 PRO A, 200088 SER A, 200175 LEU A 4.42 226.42 0.15 -0.22 1.26 86 165 200041 PRO A, 200088 SER A, 200175 LEU A, 200180 TYR A 4.23 226.95 0.02 0.3 1.25 69 166 200091 SER A, 200088 SER A, 200175 LEU A, 200180 TYR A 4.16 227.13 0.23 0.08 1.27 84 167 200091 SER A, 200088 SER A, 200180 TYR A 4.08 227.33 -0.97 -0.28 1.65 94 168 200091 SER A, 200088 SER A 4.07 227.57 -0.8 -0.97 1.67 117 169 200091 SER A, 200088 SER A, 200175 LEU A, 200180 TYR A 4.07 228.08 0.23 0.08 1.27 84 170 200088 SER A, 200091 SER A, 200041 PRO A, 200175 LEU A, 200180 TYR A 4.09 228.85 -0.06 0.08 1.67 69 171 200041 PRO A, 200175 LEU A, 200180 TYR A 4.12 233.23 0.3 0.72 1.11 54 172 200041 PRO A, 200175 LEU A, 200180 TYR A, 200173 ALA A 4.33 233.51 0.13 0.34 1.68 54 173 200041 PRO A, 200175 LEU A, 200180 TYR A 4.42 234.21 0.3 0.72 1.11 54 174 200041 PRO A, 200175 LEU A, 200180 TYR A, 200173 ALA A 4.1 234.8 0.13 0.34 1.68 54 175 200041 PRO A, 200180 TYR A, 200173 ALA A 3.66 237.01 -1.1 0.07 2.19 54 176 200041 PRO A, 200180 TYR A, 200173 ALA A, 200153 GLU A 3.39 239.27 -1.7 -0.23 14.12 61 177 200041 PRO A, 200173 ALA A, 200153 GLU A, 200172 PRO A 3.4 241.37 -1.78 -0.53 14.11 64 178 200041 PRO A, 200153 GLU A, 200172 PRO A, 200041 ASN B 3.45 241.92 -2.55 -0.52 14.11 74 179 200041 PRO A, 200153 GLU A, 200172 PRO A, 200041 ASN B 3.18 242.54 -2.55 -0.52 14.11 74 180 200153 GLU A, 200172 PRO A, 200041 ASN B 3.01 242.94 -2.87 -0.67 18.29 79 181 200173 ALA A, 200153 GLU A, 200172 PRO A, 200041 ASN B 2.4 246.36 -2.25 -0.7 14.56 79 182 200173 ALA A, 200153 GLU A, 200041 ASN B, 200172 PRO A 2.42 246.87 -1.95 -0.88 15.01 90 183 200173 ALA A, 200153 GLU A, 200041 ASN B, 200172 PRO A, 200171 PHE A 2.47 247.26 -1.64 -0.86 12.68 90 184 200153 GLU A, 200041 ASN B, 200172 PRO A, 200171 PHE A, 200182 LEU A 2.52 247.66 -0.8 -0.47 12.03 78 185 200041 ASN B, 200172 PRO A, 200171 PHE A, 200182 LEU A 2.64 248.56 -0.13 -0.31 2.57 79 186 200041 ASN B, 200171 PHE A, 200182 LEU A 2.76 248.75 -0.03 -0.14 2.3 79 187 200041 ASN B, 200182 LEU A, 200170 THR A 2.78 249.03 -0.13 -0.13 1.72 88 188 200041 ASN B, 200182 LEU A, 200170 THR A, 200155 VAL A 2.69 250.61 -0.2 -0.3 2.14 88 189 200041 ASN B, 200170 THR A, 200155 VAL A 2.8 254.19 -1.53 -0.78 2.81 105 190 200041 ASN B, 200170 THR A, 200156 THR A, 200157 VAL A 3.17 254.4 -1.33 -0.78 2.52 106 191 200041 ASN B, 200156 THR A, 200157 VAL A 3.21 254.68 -1.53 -0.78 2.81 105 192 200041 ASN B, 200170 THR A, 200156 THR A, 200157 VAL A 3.21 254.84 -1.33 -0.78 2.52 106 193 200041 ASN B, 200170 THR A, 200157 VAL A 3.19 254.95 -1.53 -0.78 2.81 105 194 200041 ASN B, 200170 THR A, 200155 VAL A 3.17 255.18 -1.53 -0.78 2.81 105 195 200041 ASN B, 200170 THR A, 200157 VAL A 3.24 255.35 -1.53 -0.78 2.81 105 196 200041 ASN B, 200170 THR A, 200156 THR A, 200157 VAL A 3.18 255.74 -1.33 -0.78 2.52 106 197 200041 ASN B, 200170 THR A, 200157 VAL A 3.21 255.89 -1.53 -0.78 2.81 105 198 200041 ASN B, 200170 THR A, 200156 THR A, 200157 VAL A 3.27 256.6 -1.33 -0.78 2.52 106 199 200041 ASN B, 200156 THR A, 200157 VAL A 3.46 259.11 -1.53 -0.78 2.81 105 200 200041 ASN B, 200156 THR A, 200157 VAL A, 200040 THR B 4.05 259.11 -1.25 -0.79 2.95 105 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
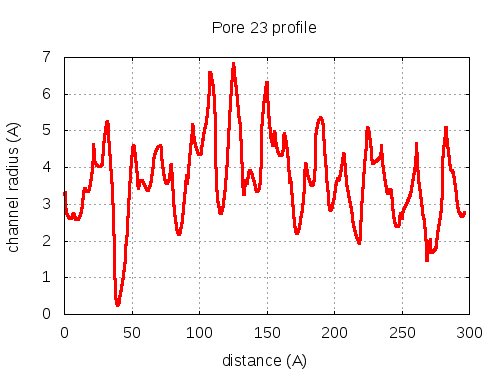
Unique lining residues set - all
100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B, 100155 VAL A, 100182 LEU A, 100171 PHE A, 100172 PRO A, 100153 GLU A, 100173 ALA A, 100172 PRO A, 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A, 100091 SER A, 100088 SER A, 100040 ARG A, 100091 SER A, 100043 HIS A, 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A, 100063 LYS A, 300020 SER B, 100003 LEU B, 100098 PHE B, 100061 ASN A, 100097 THR B, 100002 ILE B, 100001 ASP B, 300072 THR B, 300070 ASP B, 100001 ASP B, 100026 SER B, 300024 ARG B, 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B, 300028 SER B, 100027 GLN B, 300028 SER B, 100028 SER B, 300027 GLN B, 27 GLN B, 28 SER B, 200027 GLN B, 200028 SER B, 100028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 300058 GLN C, 300080 ASP C, 300079 GLY C, 300082 TYR C, 79 GLY C, 93 ARG B, 200093 ARG B, 82 TYR C, 200079 GLY C, 200082 TYR C, 100079 GLY C, 28 SER B, 200028 SER B, 69 THR B, 27 GLN B, 26 SER B, 24 ARG B, 70 ASP B, 300026 SER B, 300003 LEU B, 300001 ASP B, 72 THR B, 300001 ASP B, 300063 LYS A, 300097 THR B, 300061 ASN A, 300098 PHE B, 300046 GLU A, 300064 ILE A, 20 SER B, 300063 LYS A, 18 ARG B, 300040 ARG A, 300088 SER A, 300091 SER A, 300091 SER A, 300041 PRO A, 300088 SER A, 300175 LEU A, 300180 TYR A, 300173 ALA A, 300153 GLU A, 300172 PRO A, 300041 ASN B, 300172 PRO A, 300171 PHE A, 300182 LEU A, 300170 THR A, 300155 VAL A, 300156 THR A, 300157 VAL A, 300040 THR B
Unique lining residues set - sidechains
100156 THR A, 100170 THR A, 100041 ASN B, 100182 LEU A, 100153 GLU A, 100172 PRO A, 100041 PRO A, 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A, 100040 ARG A, 100043 HIS A, 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A, 300020 SER B, 100003 LEU B, 100061 ASN A, 100097 THR B, 100001 ASP B, 300072 THR B, 300070 ASP B, 100026 SER B, 300024 ARG B, 300069 THR B, 100027 GLN B, 300028 SER B, 300027 GLN B, 27 GLN B, 200027 GLN B, 200028 SER B, 100028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 300058 GLN C, 300082 TYR C, 93 ARG B, 200093 ARG B, 82 TYR C, 200082 TYR C, 28 SER B, 69 THR B, 24 ARG B, 70 ASP B, 300026 SER B, 300003 LEU B, 72 THR B, 300001 ASP B, 300063 LYS A, 300097 THR B, 300061 ASN A, 300046 GLU A, 300064 ILE A, 20 SER B, 18 ARG B, 300040 ARG A, 300091 SER A, 300041 PRO A, 300088 SER A, 300175 LEU A, 300180 TYR A, 300153 GLU A, 300172 PRO A, 300041 ASN B, 300182 LEU A, 300170 THR A, 300156 THR A,
Physicochemical properties of lining side-chains
Charge: 4 (12-8)
Hydropathy: -1.4
Hydrophobicity: -0.43
Polarity: 14.35
Mutability: 86
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B 3.26 0.27 -1.33 -0.78 2.52 106 2 100157 VAL A, 100170 THR A, 100041 ASN B 3.2 0.45 -1.53 -0.78 2.81 105 3 100170 THR A, 100041 ASN B, 100155 VAL A 3.19 0.62 -1.53 -0.78 2.81 105 4 100157 VAL A, 100170 THR A, 100041 ASN B 3.2 0.77 -1.53 -0.78 2.81 105 5 100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B 3.19 1.05 -1.33 -0.78 2.52 106 6 100156 THR A, 100157 VAL A, 100041 ASN B 3.04 1.59 -1.53 -0.78 2.81 105 7 100170 THR A, 100041 ASN B, 100155 VAL A 2.62 5.8 -1.53 -0.78 2.81 105 8 100170 THR A, 100041 ASN B, 100155 VAL A, 100182 LEU A 2.61 6.54 -0.2 -0.3 2.14 88 9 100170 THR A, 100041 ASN B, 100182 LEU A 2.8 6.87 -0.13 -0.13 1.72 88 10 100041 ASN B, 100182 LEU A, 100171 PHE A 2.76 7.23 -0.03 -0.14 2.3 79 11 100041 ASN B, 100182 LEU A, 100171 PHE A, 100172 PRO A 2.57 7.96 -0.13 -0.31 2.57 79 12 100041 ASN B, 100182 LEU A, 100171 PHE A, 100172 PRO A, 100153 GLU A 2.4 8.49 -0.8 -0.47 12.03 78 13 100041 ASN B, 100182 LEU A, 100172 PRO A, 100153 GLU A, 100173 ALA A 2.32 8.97 -0.8 -0.47 12.03 78 14 100041 ASN B, 100172 PRO A, 100153 GLU A, 100173 ALA A 2.26 9.54 -1.95 -0.88 15.01 90 15 100041 ASN B, 100153 GLU A, 100173 ALA A, 100172 PRO A 2.24 12.84 -2.25 -0.7 14.56 79 16 100041 ASN B, 100153 GLU A, 100172 PRO A 3.13 13.2 -2.87 -0.67 18.29 79 17 100041 ASN B, 100153 GLU A, 100172 PRO A, 100041 PRO A 3.33 13.76 -2.55 -0.52 14.11 74 18 100041 ASN B, 100153 GLU A, 100172 PRO A, 100041 PRO A 3.6 14.67 -2.55 -0.52 14.11 74 19 100153 GLU A, 100173 ALA A, 100172 PRO A, 100041 PRO A 3.4 16.96 -1.78 -0.53 14.11 64 20 100153 GLU A, 100173 ALA A, 100041 PRO A, 100180 TYR A 3.35 18.88 -1.7 -0.23 14.12 61 21 100173 ALA A, 100041 PRO A, 100180 TYR A 3.49 20.95 -1.1 0.07 2.19 54 22 100173 ALA A, 100041 PRO A, 100180 TYR A, 100175 LEU A 4.09 21.36 0.13 0.34 1.68 54 23 100041 PRO A, 100180 TYR A, 100175 LEU A 4.38 22.07 0.3 0.72 1.11 54 24 100173 ALA A, 100041 PRO A, 100180 TYR A, 100175 LEU A 4.4 22.43 0.13 0.34 1.68 54 25 100041 PRO A, 100180 TYR A, 100175 LEU A 4.3 27.21 0.3 0.72 1.11 54 26 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A, 100091 SER A 4.26 27.8 -0.06 0.08 1.67 69 27 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A 4.22 28.12 0.23 0.08 1.27 84 28 100180 TYR A, 100091 SER A, 100088 SER A 4.18 28.23 -0.97 -0.28 1.65 94 29 100091 SER A, 100088 SER A 4.17 28.32 -0.8 -0.97 1.67 117 30 100180 TYR A, 100091 SER A, 100088 SER A 4.17 28.49 -0.97 -0.28 1.65 94 31 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A 4.2 28.97 0.23 0.08 1.27 84 32 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A 4.32 29.34 0.02 0.3 1.25 69 33 100041 PRO A, 100175 LEU A, 100091 SER A, 100088 SER A 4.44 29.78 0.15 -0.22 1.26 86 34 100041 PRO A, 100091 SER A, 100088 SER A 4.58 30.75 -1.07 -0.68 1.64 97 35 100041 PRO A, 100088 SER A 4.94 31.89 -1.2 -0.53 1.63 87 36 100041 PRO A, 100088 SER A, 100040 ARG A 4.51 33.93 -2.3 -0.49 18.42 86 37 100041 PRO A, 100088 SER A, 100040 ARG A 3 36.29 -2.17 -0.44 18.99 70 38 100041 PRO A, 100088 SER A, 100040 ARG A, 100091 SER A 2.38 36.98 -1.73 -0.53 15.09 70 39 100088 SER A, 100040 ARG A, 100091 SER A 1.85 38.11 -1.77 -0.67 19.59 83 40 100088 SER A, 100040 ARG A 1.81 39.07 -2.45 -0.61 27.69 83 41 100040 ARG A 1.85 44.91 -4.5 -0.42 52 83 42 100040 ARG A, 100043 HIS A 2.59 46.14 -3.85 -0.08 51.8 87 43 100040 ARG A, 100046 GLU A 2.83 50.39 -4 -0.78 50.95 80 44 100040 ARG A, 100046 GLU A, 300018 ARG B 3.68 52.52 -4.17 -0.66 51.3 81 45 100040 ARG A, 100046 GLU A, 300018 ARG B, 100064 ILE A 3.55 56.4 -2 -0.04 38.51 86 46 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A 3.66 59.77 -0.98 -0.14 26.35 87 47 100046 GLU A, 100064 ILE A, 100063 LYS A 3.53 60.56 0.2 -0.04 17.8 90 48 100046 GLU A, 100064 ILE A, 100063 LYS A 3.32 65.06 -0.97 0.09 33.18 84 49 100046 GLU A, 100064 ILE A, 100063 LYS A, 300020 SER B 4.51 65.41 -0.93 -0.18 25.3 92 50 100046 GLU A, 100063 LYS A, 300020 SER B, 100003 LEU B 4.82 65.84 -1.1 -0.35 25.3 80 51 100046 GLU A, 100064 ILE A, 100063 LYS A, 100003 LEU B 5.06 69.97 0.23 0.35 24.92 76 52 100046 GLU A, 100063 LYS A, 100003 LEU B, 100098 PHE B 4.93 70.81 -1 -0.3 25.73 67 53 100046 GLU A, 100063 LYS A, 100003 LEU B, 100098 PHE B, 100061 ASN A 4.69 71.29 -1.5 -0.4 21.26 76 54 100046 GLU A, 100063 LYS A, 100098 PHE B, 100061 ASN A, 100097 THR B 4.43 71.46 -2.4 -0.78 21.56 90 55 100046 GLU A, 100098 PHE B, 100061 ASN A, 100097 THR B 3.94 71.67 -2.03 -0.87 14.58 96 56 100046 GLU A, 100063 LYS A, 100098 PHE B, 100061 ASN A, 100097 THR B 3.75 71.89 -2.4 -0.78 21.56 90 57 100063 LYS A, 100003 LEU B, 100098 PHE B, 100061 ASN A, 100097 THR B 3.48 72.8 -0.94 -0.32 11.61 84 58 100063 LYS A, 100003 LEU B, 100098 PHE B, 100097 THR B 3.46 73.23 -0.3 -0.21 13.67 77 59 100063 LYS A, 100003 LEU B, 100097 THR B, 100002 ILE B 3.48 73.67 -0.3 -0.21 13.67 77 60 100063 LYS A, 100003 LEU B, 100097 THR B, 100001 ASP B 3.52 75.18 -1.08 -0.27 25.25 79 61 100063 LYS A, 100003 LEU B, 100001 ASP B 3.77 76.98 -1.2 -0.1 33.11 70 62 100063 LYS A, 100003 LEU B, 100001 ASP B, 300072 THR B 4.02 77.98 -1.08 -0.27 25.25 79 63 100063 LYS A, 100003 LEU B, 100001 ASP B, 300072 THR B, 300070 ASP B 4.12 78.19 -1.56 -0.42 30.14 81 64 100003 LEU B, 100001 ASP B, 300072 THR B, 300070 ASP B 3.9 79.1 -0.98 -0.43 25.3 83 65 100003 LEU B, 300072 THR B, 300070 ASP B, 100001 ASP B 3.88 79.26 -0.2 -0.37 13.72 82 66 100003 LEU B, 300070 ASP B, 100001 ASP B 2.99 81.27 -0.03 -0.23 17.74 70 67 100003 LEU B, 300070 ASP B 2.24 84.4 0.15 0.05 24.92 70 68 100003 LEU B, 300070 ASP B, 100026 SER B 2.18 86.87 -0.17 -0.29 17.17 85 69 100003 LEU B, 300070 ASP B, 100026 SER B, 300024 ARG B 2.57 88.63 -1.25 -0.32 25.88 85 70 300070 ASP B, 100026 SER B, 300024 ARG B 3.27 90.42 -2.93 -0.81 34.46 95 71 300070 ASP B, 300024 ARG B, 100026 SER B 3.57 91.33 -2.8 -0.75 35.03 84 72 300070 ASP B, 300024 ARG B, 100026 SER B, 300069 THR B 3.78 93.35 -2.28 -0.76 26.69 92 73 300024 ARG B, 100026 SER B, 300069 THR B 4.66 94.39 -1.87 -0.66 19.01 95 74 300024 ARG B, 100026 SER B, 300069 THR B, 300026 SER B 4.6 95.6 -1.5 -0.7 15.11 95 75 100026 SER B, 300069 THR B, 300026 SER B 4.51 98.85 -0.5 -0.79 2.81 107 76 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B, 300028 SER B 4.83 101.26 -0.46 -0.79 3.04 107 77 100026 SER B, 300027 GLN B, 100027 GLN B, 300028 SER B 5.05 101.57 -1.28 -0.92 2.99 100 78 100026 SER B, 300069 THR B, 100027 GLN B, 300028 SER B 4.22 104.42 -1.35 -0.91 2.56 102 79 100026 SER B, 100027 GLN B, 300028 SER B 5.35 105.1 -1.57 -0.96 2.86 100 80 300027 GLN B, 100027 GLN B, 300028 SER B 5.66 105.75 -1.57 -0.96 2.86 100 81 100027 GLN B, 300028 SER B, 100028 SER B, 300027 GLN B 5.95 106.7 -2.05 -0.99 3.03 95 82 27 GLN B, 28 SER B, 200027 GLN B, 200028 SER B 6.41 107.65 -2.05 -0.99 3.03 95 83 100027 GLN B, 300028 SER B, 100028 SER B 5.21 111.25 -1.7 -1.01 2.29 106 84 100027 GLN B, 300028 SER B, 100028 SER B, 100093 ARG B 4.75 111.57 -2.4 -0.87 14.72 100 85 100027 GLN B, 100028 SER B, 100093 ARG B 4.4 111.97 -2.93 -0.83 19.07 94 86 100027 GLN B, 300028 SER B, 100028 SER B, 100093 ARG B 4.21 112.93 -2.4 -0.87 14.72 100 87 100027 GLN B, 300028 SER B, 100093 ARG B 2.78 118.18 -2.93 -0.83 19.07 94 88 300028 SER B, 100093 ARG B 2.79 118.9 -2.65 -0.7 26.84 100 89 300028 SER B, 100093 ARG B, 100082 TYR C 2.88 120.4 -2.2 -0.09 18.43 83 90 300028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B 3.44 120.76 -2.78 -0.18 26.82 83 91 300028 SER B, 100082 TYR C, 300093 ARG B, 300058 GLN C 3.64 121.67 -2.53 -0.35 14.7 83 92 300028 SER B, 300093 ARG B, 300058 GLN C 3.82 121.98 -2.93 -0.83 19.07 94 93 300028 SER B, 100082 TYR C, 300093 ARG B, 300058 GLN C 3.75 122.65 -2.53 -0.35 14.7 83 94 100082 TYR C, 300093 ARG B, 300058 GLN C 3.76 123.6 -3.1 -0.14 19.05 72 95 100082 TYR C, 300093 ARG B, 300058 GLN C, 300080 ASP C 3.72 123.78 -2.43 -0.3 15.13 72 96 100082 TYR C, 300093 ARG B, 300058 GLN C, 300079 GLY C 3.36 125.17 -2.43 -0.3 15.13 72 97 300093 ARG B, 300058 GLN C, 300080 ASP C, 300079 GLY C 3.43 125.8 -2.2 -0.78 15.57 83 98 300093 ARG B, 300058 GLN C, 300079 GLY C, 300082 TYR C 3.78 129.31 -2.43 -0.3 15.13 72 99 100082 TYR C, 300093 ARG B, 300079 GLY C, 300082 TYR C 5.35 130.26 -1.88 0.25 14.65 61 100 100082 TYR C, 300093 ARG B, 300079 GLY C, 300082 TYR C, 79 GLY C 5.67 131.52 -1.58 0.04 12.4 61 101 93 ARG B, 200093 ARG B, 82 TYR C, 200079 GLY C, 200082 TYR C 6.25 131.75 -2.4 0.12 22.12 66 102 100093 ARG B, 200093 ARG B, 82 TYR C, 200079 GLY C, 200082 TYR C 6.47 132.16 -2.4 0.12 22.12 66 103 200093 ARG B, 82 TYR C, 200079 GLY C, 200082 TYR C, 100079 GLY C 6.78 132.45 -1.58 0.04 12.4 61 104 300093 ARG B, 300079 GLY C, 300082 TYR C, 79 GLY C, 93 ARG B 6.44 133.57 -2.22 -0.27 22.47 72 105 300093 ARG B, 300082 TYR C, 79 GLY C, 93 ARG B, 28 SER B 6.21 134.01 -2.3 -0.3 22.13 83 106 300093 ARG B, 300082 TYR C, 93 ARG B, 28 SER B 3.95 137.11 -2.78 -0.18 26.82 83 107 300093 ARG B, 300082 TYR C, 28 SER B 3.25 139.15 -2.2 -0.09 18.43 83 108 300093 ARG B, 28 SER B 3.13 139.88 -2.65 -0.7 26.84 100 109 300027 GLN B, 300093 ARG B, 28 SER B 3.07 144.46 -2.93 -0.83 19.07 94 110 300028 SER B, 300027 GLN B, 300093 ARG B, 28 SER B 3.84 144.88 -2.3 -0.82 15.15 94 111 300028 SER B, 300027 GLN B, 300093 ARG B, 28 SER B 4.05 145.08 -2.4 -0.87 14.72 100 112 300028 SER B, 300027 GLN B, 300093 ARG B 4.27 145.42 -2.93 -0.83 19.07 94 113 300028 SER B, 300027 GLN B, 300093 ARG B, 28 SER B 4.94 145.92 -2.4 -0.87 14.72 100 114 300028 SER B, 300027 GLN B, 28 SER B 5.35 149.53 -1.7 -1.01 2.29 106 115 100027 GLN B, 200027 GLN B, 100028 SER B, 200028 SER B 6.44 150.46 -2.05 -0.99 3.03 95 116 300028 SER B, 300027 GLN B, 27 GLN B, 28 SER B 6.24 151.6 -2.05 -0.99 3.03 95 117 300027 GLN B, 27 GLN B, 28 SER B 5.9 152.28 -2.6 -1.06 2.91 95 118 300026 SER B, 300027 GLN B, 28 SER B 5.5 152.94 -1.57 -0.96 2.86 100 119 300026 SER B, 300027 GLN B, 28 SER B, 69 THR B 4.14 155.6 -1.35 -0.91 2.56 102 120 300026 SER B, 300027 GLN B, 28 SER B, 27 GLN B 5.1 155.81 -1.28 -0.92 2.99 100 121 300026 SER B, 28 SER B, 69 THR B, 27 GLN B, 26 SER B 4.51 158.73 -0.46 -0.79 3.04 107 122 300026 SER B, 69 THR B, 27 GLN B, 26 SER B 4.38 159.68 -0.48 -0.79 2.95 107 123 300026 SER B, 69 THR B, 26 SER B 4.32 161.57 -0.5 -0.79 2.81 107 124 300026 SER B, 69 THR B, 26 SER B, 24 ARG B 4.64 162.83 -1.5 -0.7 15.11 95 125 300026 SER B, 69 THR B, 24 ARG B 4.39 164.56 -1.87 -0.66 19.01 95 126 300026 SER B, 69 THR B, 24 ARG B, 70 ASP B 3.71 165.77 -2.28 -0.76 26.69 92 127 300026 SER B, 24 ARG B, 70 ASP B 3.59 166.51 -2.8 -0.75 35.03 84 128 24 ARG B, 70 ASP B, 300026 SER B 3.22 168.58 -2.93 -0.81 34.46 95 129 24 ARG B, 70 ASP B, 300026 SER B, 300003 LEU B 2.46 170.38 -1.25 -0.32 25.88 85 130 70 ASP B, 300026 SER B, 300003 LEU B 2.09 173.4 -0.17 -0.29 17.17 85 131 70 ASP B, 300003 LEU B 2.29 175.94 0.15 0.05 24.92 70 132 70 ASP B, 300003 LEU B, 300001 ASP B 3.03 177.91 -0.03 -0.23 17.74 70 133 70 ASP B, 300003 LEU B, 72 THR B, 300001 ASP B 3.83 178.86 -0.98 -0.43 25.3 83 134 70 ASP B, 300003 LEU B, 72 THR B, 300001 ASP B, 300063 LYS A 4.19 179.08 -1.56 -0.42 30.14 81 135 300003 LEU B, 72 THR B, 300001 ASP B, 300063 LYS A 3.99 180.32 -1.08 -0.27 25.25 79 136 300003 LEU B, 300001 ASP B, 300063 LYS A 3.67 182.13 -1.2 -0.1 33.11 70 137 300003 LEU B, 300001 ASP B, 300063 LYS A, 300097 THR B 3.53 183.98 -1.08 -0.27 25.25 79 138 300003 LEU B, 300063 LYS A, 300097 THR B, 300061 ASN A 3.63 184.38 -1.08 -0.2 13.67 84 139 300003 LEU B, 300063 LYS A, 300097 THR B, 300061 ASN A, 300098 PHE B 3.72 185.17 -0.94 -0.32 11.61 84 140 300063 LYS A, 300097 THR B, 300061 ASN A, 300098 PHE B, 300046 GLU A 4.07 185.36 -2.4 -0.78 21.56 90 141 300097 THR B, 300061 ASN A, 300098 PHE B, 300046 GLU A 4.19 185.93 -2.03 -0.87 14.58 96 142 300063 LYS A, 300097 THR B, 300061 ASN A, 300098 PHE B, 300046 GLU A 4.48 186.67 -2.4 -0.78 21.56 90 143 300003 LEU B, 300063 LYS A, 300061 ASN A, 300098 PHE B, 300046 GLU A 4.54 187.7 -1.5 -0.4 21.26 76 144 300003 LEU B, 300063 LYS A, 300098 PHE B, 300046 GLU A 4.57 188.86 -1 -0.3 25.73 67 145 300003 LEU B, 300063 LYS A, 300046 GLU A, 300064 ILE A 4.47 191.42 0.23 0.35 24.92 76 146 300003 LEU B, 300063 LYS A, 300046 GLU A, 20 SER B 4.29 192.26 -1.1 -0.35 25.3 80 147 300063 LYS A, 300046 GLU A, 300064 ILE A, 20 SER B 4.19 193.01 -0.93 -0.18 25.3 92 148 300063 LYS A, 300046 GLU A, 300064 ILE A 3.81 197.71 -0.97 0.09 33.18 84 149 300046 GLU A, 300064 ILE A, 300063 LYS A, 18 ARG B 3.74 200.92 -0.98 -0.14 26.35 87 150 300046 GLU A, 300064 ILE A, 18 ARG B, 300040 ARG A 3.58 204.19 -2 -0.04 38.51 86 151 300046 GLU A, 18 ARG B, 300040 ARG A 3.91 206.96 -4.17 -0.66 51.3 81 152 300046 GLU A, 300064 ILE A, 300040 ARG A 4.12 207.47 -1.17 0.08 34.01 87 153 300046 GLU A, 300040 ARG A 2.19 210.96 -4 -0.78 50.95 80 154 300040 ARG A 0.21 217.1 -4.5 -0.42 52 83 155 300040 ARG A, 300088 SER A 0.25 217.86 -2.45 -0.61 27.69 83 156 300040 ARG A, 300088 SER A, 300091 SER A 0.5 218.27 -1.77 -0.67 19.59 83 157 300040 ARG A, 300088 SER A, 300091 SER A 0.92 218.51 -1.9 -0.73 19.02 100 158 300040 ARG A, 300088 SER A, 300091 SER A, 300041 PRO A 1.45 218.78 -1.83 -0.57 14.66 86 159 300040 ARG A, 300088 SER A, 300091 SER A, 300041 PRO A 2.05 219.53 -1.73 -0.53 15.09 70 160 300040 ARG A, 300088 SER A, 300041 PRO A 3.24 222.04 -2.17 -0.44 18.99 70 161 300040 ARG A, 300041 PRO A, 300088 SER A 4.9 224.17 -2.3 -0.49 18.42 86 162 300041 PRO A, 300088 SER A 4.86 225.08 -1.2 -0.53 1.63 87 163 300091 SER A, 300041 PRO A, 300088 SER A 4.55 226.03 -1.07 -0.68 1.64 97 164 300091 SER A, 300041 PRO A, 300088 SER A, 300175 LEU A 4.42 226.42 0.15 -0.22 1.26 86 165 300041 PRO A, 300088 SER A, 300175 LEU A, 300180 TYR A 4.23 226.95 0.02 0.3 1.25 69 166 300091 SER A, 300088 SER A, 300175 LEU A, 300180 TYR A 4.16 227.13 0.23 0.08 1.27 84 167 300091 SER A, 300088 SER A, 300180 TYR A 4.08 227.32 -0.97 -0.28 1.65 94 168 300091 SER A, 300088 SER A 4.07 227.57 -0.8 -0.97 1.67 117 169 300091 SER A, 300088 SER A, 300175 LEU A, 300180 TYR A 4.07 228.08 0.23 0.08 1.27 84 170 300088 SER A, 300091 SER A, 300041 PRO A, 300175 LEU A, 300180 TYR A 4.09 228.85 -0.06 0.08 1.67 69 171 300041 PRO A, 300175 LEU A, 300180 TYR A 4.12 233.23 0.3 0.72 1.11 54 172 300041 PRO A, 300175 LEU A, 300180 TYR A, 300173 ALA A 4.33 233.51 0.13 0.34 1.68 54 173 300041 PRO A, 300175 LEU A, 300180 TYR A 4.42 234.21 0.3 0.72 1.11 54 174 300041 PRO A, 300175 LEU A, 300180 TYR A, 300173 ALA A 4.1 234.79 0.13 0.34 1.68 54 175 300041 PRO A, 300180 TYR A, 300173 ALA A 3.66 237.01 -1.1 0.07 2.19 54 176 300041 PRO A, 300180 TYR A, 300173 ALA A, 300153 GLU A 3.39 239.27 -1.7 -0.23 14.12 61 177 300041 PRO A, 300173 ALA A, 300153 GLU A, 300172 PRO A 3.4 241.37 -1.78 -0.53 14.11 64 178 300041 PRO A, 300153 GLU A, 300172 PRO A, 300041 ASN B 3.45 241.92 -2.55 -0.52 14.11 74 179 300041 PRO A, 300153 GLU A, 300172 PRO A, 300041 ASN B 3.18 242.54 -2.55 -0.52 14.11 74 180 300153 GLU A, 300172 PRO A, 300041 ASN B 3.01 242.93 -2.87 -0.67 18.29 79 181 300173 ALA A, 300153 GLU A, 300172 PRO A, 300041 ASN B 2.4 246.36 -2.25 -0.7 14.56 79 182 300173 ALA A, 300153 GLU A, 300041 ASN B, 300172 PRO A 2.42 246.87 -1.95 -0.88 15.01 90 183 300173 ALA A, 300153 GLU A, 300041 ASN B, 300172 PRO A, 300171 PHE A 2.47 247.26 -1.64 -0.86 12.68 90 184 300153 GLU A, 300041 ASN B, 300172 PRO A, 300171 PHE A, 300182 LEU A 2.52 247.66 -0.8 -0.47 12.03 78 185 300041 ASN B, 300172 PRO A, 300171 PHE A, 300182 LEU A 2.64 248.56 -0.13 -0.31 2.57 79 186 300041 ASN B, 300171 PHE A, 300182 LEU A 2.76 248.75 -0.03 -0.14 2.3 79 187 300041 ASN B, 300182 LEU A, 300170 THR A 2.78 249.03 -0.13 -0.13 1.72 88 188 300041 ASN B, 300182 LEU A, 300170 THR A, 300155 VAL A 2.69 250.61 -0.2 -0.3 2.14 88 189 300041 ASN B, 300170 THR A, 300155 VAL A 2.8 254.19 -1.53 -0.78 2.81 105 190 300041 ASN B, 300170 THR A, 300156 THR A, 300157 VAL A 3.17 254.4 -1.33 -0.78 2.52 106 191 300041 ASN B, 300156 THR A, 300157 VAL A 3.21 254.68 -1.53 -0.78 2.81 105 192 300041 ASN B, 300170 THR A, 300156 THR A, 300157 VAL A 3.21 254.84 -1.33 -0.78 2.52 106 193 300041 ASN B, 300170 THR A, 300157 VAL A 3.19 254.95 -1.53 -0.78 2.81 105 194 300041 ASN B, 300170 THR A, 300155 VAL A 3.17 255.18 -1.53 -0.78 2.81 105 195 300041 ASN B, 300170 THR A, 300157 VAL A 3.24 255.35 -1.53 -0.78 2.81 105 196 300041 ASN B, 300170 THR A, 300156 THR A, 300157 VAL A 3.18 255.74 -1.33 -0.78 2.52 106 197 300041 ASN B, 300170 THR A, 300157 VAL A 3.21 255.89 -1.53 -0.78 2.81 105 198 300041 ASN B, 300170 THR A, 300156 THR A, 300157 VAL A 3.27 256.6 -1.33 -0.78 2.52 106 199 300041 ASN B, 300156 THR A, 300157 VAL A 3.46 259.11 -1.53 -0.78 2.81 105 200 300041 ASN B, 300156 THR A, 300157 VAL A, 300040 THR B 4.05 259.11 -1.25 -0.79 2.95 105 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
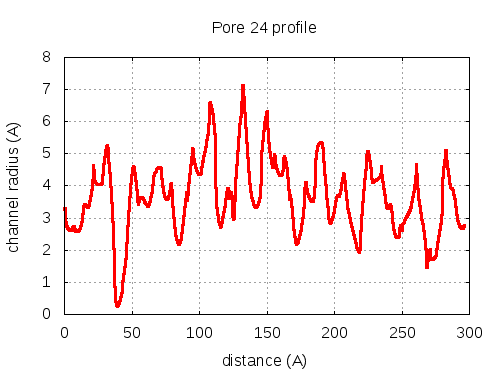
Unique lining residues set - all
100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B, 100155 VAL A, 100182 LEU A, 100171 PHE A, 100172 PRO A, 100153 GLU A, 100173 ALA A, 100172 PRO A, 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A, 100091 SER A, 100088 SER A, 100040 ARG A, 100091 SER A, 100043 HIS A, 100046 GLU A, 100064 ILE A, 300018 ARG B, 100063 LYS A, 100063 LYS A, 300020 SER B, 100003 LEU B, 100098 PHE B, 100061 ASN A, 100097 THR B, 100002 ILE B, 100001 ASP B, 300072 THR B, 300070 ASP B, 100001 ASP B, 100026 SER B, 300024 ARG B, 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B, 300028 SER B, 100027 GLN B, 300028 SER B, 100028 SER B, 300027 GLN B, 27 GLN B, 28 SER B, 200027 GLN B, 200028 SER B, 100028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 300079 GLY C, 100079 GLY C, 93 ARG B, 200093 ARG B, 79 GLY C, 82 TYR C, 200079 GLY C, 300082 TYR C, 200082 TYR C, 100058 GLN C, 100080 ASP C, 28 SER B, 200058 GLN C, 200080 ASP C, 200027 GLN B, 26 SER B, 200069 THR B, 200026 SER B, 200028 SER B, 200024 ARG B, 200070 ASP B, 26 SER B, 3 LEU B, 1 ASP B, 200072 THR B, 1 ASP B, 63 LYS A, 97 THR B, 2 ILE B, 98 PHE B, 61 ASN A, 46 GLU A, 64 ILE A, 200020 SER B, 63 LYS A, 200018 ARG B, 40 ARG A, 88 SER A, 91 SER A, 91 SER A, 41 PRO A, 88 SER A, 175 LEU A, 180 TYR A, 173 ALA A, 153 GLU A, 172 PRO A, 41 ASN B, 42 GLY A, 165 ASP B, 40 THR B, 85 ASN B, 103 LYS B, 10 ILE B
Unique lining residues set - sidechains
100156 THR A, 100170 THR A, 100041 ASN B, 100182 LEU A, 100153 GLU A, 100172 PRO A, 100041 PRO A, 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A, 100040 ARG A, 100043 HIS A, 100046 GLU A, 100064 ILE A, 300018 ARG B, 100063 LYS A, 300020 SER B, 100003 LEU B, 100061 ASN A, 100097 THR B, 100001 ASP B, 300072 THR B, 300070 ASP B, 100026 SER B, 300024 ARG B, 300069 THR B, 100027 GLN B, 300028 SER B, 300027 GLN B, 27 GLN B, 200027 GLN B, 200028 SER B, 100028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 93 ARG B, 200093 ARG B, 82 TYR C, 300082 TYR C, 200082 TYR C, 100058 GLN C, 28 SER B, 200058 GLN C, 200069 THR B, 200024 ARG B, 200070 ASP B, 26 SER B, 3 LEU B, 200072 THR B, 1 ASP B, 63 LYS A, 97 THR B, 61 ASN A, 46 GLU A, 64 ILE A, 200020 SER B, 200018 ARG B, 40 ARG A, 91 SER A, 41 PRO A, 88 SER A, 175 LEU A, 180 TYR A, 153 GLU A, 172 PRO A, 41 ASN B, 165 ASP B, 40 THR B, 85 ASN B, 103 LYS B, 10 ILE B
Physicochemical properties of lining side-chains
Charge: 4 (13-9)
Hydropathy: -1.5
Hydrophobicity: -0.41
Polarity: 15.58
Mutability: 87
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B 3.26 0.25 -1.33 -0.78 2.52 106 2 100157 VAL A, 100170 THR A, 100041 ASN B 3.21 0.43 -1.53 -0.78 2.81 105 3 100170 THR A, 100041 ASN B, 100155 VAL A 3.19 0.68 -1.53 -0.78 2.81 105 4 100157 VAL A, 100170 THR A, 100041 ASN B 3.2 0.83 -1.53 -0.78 2.81 105 5 100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B 3.2 0.98 -1.33 -0.78 2.52 106 6 100156 THR A, 100157 VAL A, 100041 ASN B 3.11 1.3 -1.53 -0.78 2.81 105 7 100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B 3.05 1.73 -1.33 -0.78 2.52 106 8 100170 THR A, 100041 ASN B, 100155 VAL A 2.69 5.4 -1.53 -0.78 2.81 105 9 100170 THR A, 100041 ASN B, 100155 VAL A, 100182 LEU A 2.65 6.67 -0.2 -0.3 2.14 88 10 100170 THR A, 100041 ASN B, 100182 LEU A 2.8 6.84 -0.13 -0.13 1.72 88 11 100041 ASN B, 100182 LEU A, 100171 PHE A 2.76 7.2 -0.03 -0.14 2.3 79 12 100041 ASN B, 100182 LEU A, 100171 PHE A, 100172 PRO A 2.62 7.91 -0.13 -0.31 2.57 79 13 100041 ASN B, 100182 LEU A, 100171 PHE A, 100172 PRO A, 100153 GLU A 2.41 8.64 -0.8 -0.47 12.03 78 14 100041 ASN B, 100171 PHE A, 100172 PRO A, 100153 GLU A, 100173 ALA A 2.34 9.13 -1.64 -0.86 12.68 90 15 100041 ASN B, 100172 PRO A, 100153 GLU A, 100173 ALA A 2.29 9.71 -1.95 -0.88 15.01 90 16 100041 ASN B, 100153 GLU A, 100173 ALA A, 100172 PRO A 2.27 12.88 -2.25 -0.7 14.56 79 17 100041 ASN B, 100153 GLU A, 100172 PRO A 3.09 13.22 -2.87 -0.67 18.29 79 18 100041 ASN B, 100153 GLU A, 100172 PRO A, 100041 PRO A 3.29 13.76 -2.55 -0.52 14.11 74 19 100041 ASN B, 100153 GLU A, 100172 PRO A, 100041 PRO A 3.58 14.64 -2.55 -0.52 14.11 74 20 100153 GLU A, 100173 ALA A, 100172 PRO A, 100041 PRO A 3.44 16.83 -1.78 -0.53 14.11 64 21 100153 GLU A, 100173 ALA A, 100041 PRO A, 100180 TYR A 3.41 18.64 -1.7 -0.23 14.12 61 22 100173 ALA A, 100041 PRO A, 100180 TYR A 3.52 21.06 -1.1 0.07 2.19 54 23 100173 ALA A, 100041 PRO A, 100180 TYR A, 100175 LEU A 4.1 21.4 0.13 0.34 1.68 54 24 100041 PRO A, 100180 TYR A, 100175 LEU A 4.4 22.08 0.3 0.72 1.11 54 25 100173 ALA A, 100041 PRO A, 100180 TYR A, 100175 LEU A 4.37 22.41 0.13 0.34 1.68 54 26 100041 PRO A, 100180 TYR A, 100175 LEU A 4.19 27 0.3 0.72 1.11 54 27 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A, 100091 SER A 4.15 27.66 -0.06 0.08 1.67 69 28 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A 4.11 28.05 0.02 0.3 1.25 69 29 100180 TYR A, 100091 SER A, 100088 SER A 4.09 28.21 -0.97 -0.28 1.65 94 30 100091 SER A, 100088 SER A 4.08 28.28 -0.8 -0.97 1.67 117 31 100180 TYR A, 100091 SER A, 100088 SER A 4.08 28.6 -0.97 -0.28 1.65 94 32 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A 4.16 28.84 0.23 0.08 1.27 84 33 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A 4.23 29.53 0.02 0.3 1.25 69 34 100041 PRO A, 100175 LEU A, 100091 SER A, 100088 SER A 4.44 29.98 0.15 -0.22 1.26 86 35 100041 PRO A, 100091 SER A, 100088 SER A 4.57 30.45 -1.07 -0.68 1.64 97 36 100041 PRO A, 100088 SER A 4.73 31.69 -1.2 -0.53 1.63 87 37 100041 PRO A, 100088 SER A, 100040 ARG A 4.86 33.98 -2.3 -0.49 18.42 86 38 100041 PRO A, 100088 SER A, 100040 ARG A 3.32 36.27 -2.17 -0.44 18.99 70 39 100041 PRO A, 100088 SER A, 100040 ARG A, 100091 SER A 2.33 36.95 -1.73 -0.53 15.09 70 40 100088 SER A, 100040 ARG A, 100091 SER A 1.02 37.96 -1.77 -0.67 19.59 83 41 100088 SER A, 100040 ARG A 0.63 39.99 -2.45 -0.61 27.69 83 42 100040 ARG A 0.67 45.68 -4.5 -0.42 52 83 43 100040 ARG A, 100043 HIS A 2.2 46.73 -3.85 -0.08 51.8 87 44 100040 ARG A, 100043 HIS A, 100046 GLU A 2.7 47.61 -3.73 -0.43 51.17 83 45 100040 ARG A, 100046 GLU A 3.16 50.01 -4 -0.78 50.95 80 46 100040 ARG A, 100046 GLU A, 100064 ILE A 3.91 50.65 -1.17 0.08 34.01 87 47 100040 ARG A, 100046 GLU A, 300018 ARG B 3.76 52.69 -4.17 -0.66 51.3 81 48 100040 ARG A, 100046 GLU A, 100064 ILE A, 300018 ARG B 3.56 56.38 -2 -0.04 38.51 86 49 100046 GLU A, 100064 ILE A, 300018 ARG B, 100063 LYS A 3.69 59.61 -0.98 -0.14 26.35 87 50 100046 GLU A, 100064 ILE A, 100063 LYS A 3.79 60.38 0.2 -0.04 17.8 90 51 100046 GLU A, 100064 ILE A, 100063 LYS A 3.83 64.82 -0.97 0.09 33.18 84 52 100046 GLU A, 100064 ILE A, 100063 LYS A, 300020 SER B 4.26 65.29 -0.93 -0.18 25.3 92 53 100046 GLU A, 100063 LYS A, 300020 SER B, 100003 LEU B 4.32 66.16 -1.1 -0.35 25.3 80 54 100046 GLU A, 100064 ILE A, 100063 LYS A, 100003 LEU B 4.42 69.28 0.23 0.35 24.92 76 55 100046 GLU A, 100063 LYS A, 100003 LEU B, 100098 PHE B 4.48 70.27 -1 -0.3 25.73 67 56 100046 GLU A, 100063 LYS A, 100003 LEU B, 100098 PHE B, 100061 ASN A 4.46 70.99 -1.5 -0.4 21.26 76 57 100046 GLU A, 100063 LYS A, 100098 PHE B, 100061 ASN A, 100097 THR B 4.42 71.37 -2.4 -0.78 21.56 90 58 100046 GLU A, 100098 PHE B, 100061 ASN A, 100097 THR B 4.24 71.73 -2.03 -0.87 14.58 96 59 100046 GLU A, 100063 LYS A, 100098 PHE B, 100061 ASN A, 100097 THR B 4.15 71.94 -2.4 -0.78 21.56 90 60 100063 LYS A, 100003 LEU B, 100098 PHE B, 100061 ASN A, 100097 THR B 3.85 72.84 -0.94 -0.32 11.61 84 61 100063 LYS A, 100003 LEU B, 100098 PHE B, 100097 THR B 3.76 73.25 -0.3 -0.21 13.67 77 62 100063 LYS A, 100003 LEU B, 100097 THR B, 100002 ILE B 3.68 73.68 -0.3 -0.21 13.67 77 63 100063 LYS A, 100003 LEU B, 100097 THR B, 100001 ASP B 3.59 75.13 -1.08 -0.27 25.25 79 64 100063 LYS A, 100003 LEU B, 100001 ASP B 3.64 77.35 -1.2 -0.1 33.11 70 65 100063 LYS A, 100003 LEU B, 100001 ASP B, 300072 THR B 3.96 78.14 -1.08 -0.27 25.25 79 66 100063 LYS A, 100003 LEU B, 100001 ASP B, 300072 THR B, 300070 ASP B 4.14 78.34 -1.56 -0.42 30.14 81 67 100003 LEU B, 100001 ASP B, 300072 THR B, 300070 ASP B 3.82 79.17 -0.98 -0.43 25.3 83 68 100003 LEU B, 300070 ASP B, 100001 ASP B 3.1 81.24 -0.03 -0.23 17.74 70 69 100003 LEU B, 300070 ASP B 2.22 84.27 0.15 0.05 24.92 70 70 100003 LEU B, 300070 ASP B, 100026 SER B 2.11 87.14 -0.17 -0.29 17.17 85 71 100003 LEU B, 300070 ASP B, 100026 SER B, 300024 ARG B 2.49 88.82 -1.25 -0.32 25.88 85 72 300070 ASP B, 100026 SER B, 300024 ARG B 3.21 90.45 -2.93 -0.81 34.46 95 73 300070 ASP B, 300024 ARG B, 100026 SER B 3.67 91.34 -2.8 -0.75 35.03 84 74 300070 ASP B, 300024 ARG B, 100026 SER B, 300069 THR B 3.7 93.27 -2.28 -0.76 26.69 92 75 300024 ARG B, 100026 SER B, 300069 THR B 4.36 94.66 -1.87 -0.66 19.01 95 76 300024 ARG B, 100026 SER B, 300069 THR B, 300026 SER B 4.91 95.45 -1.5 -0.7 15.11 95 77 100026 SER B, 300069 THR B, 300026 SER B 4.34 98.33 -0.5 -0.79 2.81 107 78 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B 4.32 99.25 -0.48 -0.79 2.95 107 79 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B, 300028 SER B 4.38 101.33 -0.46 -0.79 3.04 107 80 100026 SER B, 300027 GLN B, 100027 GLN B, 300028 SER B 4.92 101.65 -1.28 -0.92 2.99 100 81 100026 SER B, 300069 THR B, 100027 GLN B, 300028 SER B 4.23 104.38 -1.35 -0.91 2.56 102 82 100026 SER B, 100027 GLN B, 300028 SER B 5.09 105.04 -1.57 -0.96 2.86 100 83 300027 GLN B, 100027 GLN B, 300028 SER B 5.47 105.68 -1.57 -0.96 2.86 100 84 100027 GLN B, 300028 SER B, 100028 SER B, 300027 GLN B 5.84 106.66 -2.05 -0.99 3.03 95 85 27 GLN B, 28 SER B, 200027 GLN B, 200028 SER B 6.32 107.52 -2.05 -0.99 3.03 95 86 200027 GLN B, 200028 SER B, 100028 SER B 6.14 107.98 -1.7 -1.01 2.29 106 87 100027 GLN B, 300028 SER B, 100028 SER B 5.37 111.37 -1.7 -1.01 2.29 106 88 100027 GLN B, 300028 SER B, 100028 SER B, 100093 ARG B 5.17 111.63 -2.4 -0.87 14.72 100 89 100027 GLN B, 100028 SER B, 100093 ARG B 4.5 112.04 -2.93 -0.83 19.07 94 90 100027 GLN B, 300028 SER B, 100028 SER B, 100093 ARG B 3.83 113.02 -2.4 -0.87 14.72 100 91 100027 GLN B, 300028 SER B, 100093 ARG B 2.76 118.27 -2.93 -0.83 19.07 94 92 300028 SER B, 100093 ARG B 3.34 118.98 -2.65 -0.7 26.84 100 93 300028 SER B, 100093 ARG B, 100082 TYR C 3.6 120.09 -2.2 -0.09 18.43 83 94 300028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B 4.15 123.08 -2.78 -0.18 26.82 83 95 300028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 300079 GLY C 5.93 123.89 -2.3 -0.3 22.13 83 96 100093 ARG B, 100082 TYR C, 300093 ARG B, 300079 GLY C, 100079 GLY C 6.33 124.81 -2.22 -0.27 22.47 72 97 93 ARG B, 200093 ARG B, 79 GLY C, 82 TYR C, 200079 GLY C 6.62 125 -2.22 -0.27 22.47 72 98 93 ARG B, 200093 ARG B, 79 GLY C, 82 TYR C, 300082 TYR C 6.25 125.39 -2.4 0.12 22.12 66 99 300093 ARG B, 93 ARG B, 79 GLY C, 82 TYR C, 300082 TYR C 5.97 125.85 -2.4 0.12 22.12 66 100 100093 ARG B, 100082 TYR C, 300079 GLY C, 100079 GLY C, 200082 TYR C 4.92 128.37 -1.58 0.04 12.4 61 101 100093 ARG B, 100082 TYR C, 100079 GLY C, 100058 GLN C 3.42 131.62 -2.43 -0.3 15.13 72 102 100093 ARG B, 100079 GLY C, 100058 GLN C, 100080 ASP C 3.29 132.8 -2.2 -0.78 15.57 83 103 100093 ARG B, 100079 GLY C, 200082 TYR C, 100058 GLN C 3.63 135.1 -2.43 -0.3 15.13 72 104 100093 ARG B, 100082 TYR C, 100079 GLY C, 200082 TYR C, 100058 GLN C 5 135.99 -2.2 -0.02 12.43 66 105 100093 ARG B, 100082 TYR C, 100079 GLY C, 200093 ARG B, 200082 TYR C 5.54 136.84 -2.4 0.12 22.12 66 106 100093 ARG B, 100079 GLY C, 200093 ARG B, 200079 GLY C, 200082 TYR C 6.05 138.12 -2.22 -0.27 22.47 72 107 300093 ARG B, 300079 GLY C, 93 ARG B, 79 GLY C, 300082 TYR C 6.8 138.67 -2.22 -0.27 22.47 72 108 300093 ARG B, 300079 GLY C, 93 ARG B, 300082 TYR C, 28 SER B 6.89 138.96 -2.3 -0.3 22.13 83 109 100082 TYR C, 300093 ARG B, 300079 GLY C, 93 ARG B, 300082 TYR C 6.69 139.37 -2.4 0.12 22.12 66 110 100093 ARG B, 100082 TYR C, 300093 ARG B, 300079 GLY C, 300082 TYR C 6.41 140.02 -2.4 0.12 22.12 66 111 100079 GLY C, 200093 ARG B, 82 TYR C, 200079 GLY C, 200082 TYR C 5.7 141.84 -1.58 0.04 12.4 61 112 200093 ARG B, 82 TYR C, 200079 GLY C, 200082 TYR C 5.31 142.8 -1.88 0.25 14.65 61 113 200093 ARG B, 200079 GLY C, 200082 TYR C, 200058 GLN C 3.63 145.28 -2.43 -0.3 15.13 72 114 200093 ARG B, 200079 GLY C, 200058 GLN C, 200080 ASP C 3.33 145.98 -2.2 -0.78 15.57 83 115 200093 ARG B, 82 TYR C, 200079 GLY C, 200058 GLN C 3.29 147.11 -2.43 -0.3 15.13 72 116 200093 ARG B, 82 TYR C, 200058 GLN C, 200080 ASP C 3.76 147.29 -2.43 -0.3 15.13 72 117 200093 ARG B, 82 TYR C, 200058 GLN C 3.8 148.27 -3.1 -0.14 19.05 72 118 200028 SER B, 200093 ARG B, 82 TYR C, 200058 GLN C 3.78 148.83 -2.53 -0.35 14.7 83 119 200028 SER B, 200093 ARG B, 200058 GLN C 3.83 149.24 -2.93 -0.83 19.07 94 120 200028 SER B, 200093 ARG B, 82 TYR C, 200058 GLN C 3.54 150.47 -2.53 -0.35 14.7 83 121 200028 SER B, 93 ARG B, 200093 ARG B, 82 TYR C 3.35 150.88 -2.78 -0.18 26.82 83 122 200028 SER B, 93 ARG B, 82 TYR C 2.86 152.75 -2.2 -0.09 18.43 83 123 200028 SER B, 93 ARG B 2.79 153.47 -2.65 -0.7 26.84 100 124 27 GLN B, 200028 SER B, 93 ARG B 2.81 158.38 -2.93 -0.83 19.07 94 125 27 GLN B, 200028 SER B, 93 ARG B, 28 SER B 4.21 158.91 -2.4 -0.87 14.72 100 126 27 GLN B, 93 ARG B, 28 SER B 4.39 159.25 -2.93 -0.83 19.07 94 127 27 GLN B, 200028 SER B, 93 ARG B, 28 SER B 4.72 159.76 -2.4 -0.87 14.72 100 128 27 GLN B, 200028 SER B, 28 SER B 5.14 163.3 -1.7 -1.01 2.29 106 129 100027 GLN B, 300028 SER B, 100028 SER B, 300027 GLN B 6.28 164.61 -2.05 -0.99 3.03 95 130 27 GLN B, 28 SER B, 200027 GLN B, 200028 SER B 6.07 165.19 -2.05 -0.99 3.03 95 131 27 GLN B, 200027 GLN B, 200028 SER B 5.82 165.84 -2.6 -1.06 2.91 95 132 27 GLN B, 200028 SER B, 200027 GLN B 5.54 166.49 -1.57 -0.96 2.86 100 133 27 GLN B, 200028 SER B, 26 SER B 5.25 167.08 -1.57 -0.96 2.86 100 134 27 GLN B, 200028 SER B, 26 SER B, 200069 THR B 4.25 169.31 -1.35 -0.91 2.56 102 135 27 GLN B, 200028 SER B, 200027 GLN B, 26 SER B 5.01 169.69 -1.28 -0.92 2.99 100 136 200027 GLN B, 26 SER B, 200069 THR B, 200026 SER B, 200028 SER B 4.95 172.61 -0.46 -0.79 3.04 107 137 200027 GLN B, 26 SER B, 200069 THR B, 200026 SER B 4.83 173.52 -0.48 -0.79 2.95 107 138 26 SER B, 200069 THR B, 200026 SER B 4.58 175.62 -0.5 -0.79 2.81 107 139 26 SER B, 200069 THR B, 200026 SER B, 200024 ARG B 4.65 176.53 -1.5 -0.7 15.11 95 140 26 SER B, 200069 THR B, 200024 ARG B 4.58 178.17 -1.87 -0.66 19.01 95 141 26 SER B, 200069 THR B, 200024 ARG B, 200070 ASP B 3.96 179.46 -2.28 -0.76 26.69 92 142 26 SER B, 200024 ARG B, 200070 ASP B 3.57 180.34 -2.8 -0.75 35.03 84 143 200024 ARG B, 200070 ASP B, 26 SER B 3.38 182.43 -2.93 -0.81 34.46 95 144 200024 ARG B, 200070 ASP B, 26 SER B, 3 LEU B 2.67 184.18 -1.25 -0.32 25.88 85 145 200070 ASP B, 26 SER B, 3 LEU B 2.11 187.1 -0.17 -0.29 17.17 85 146 200070 ASP B, 3 LEU B 2.22 189.6 0.15 0.05 24.92 70 147 200070 ASP B, 3 LEU B, 1 ASP B 2.93 191.6 -0.03 -0.23 17.74 70 148 200070 ASP B, 3 LEU B, 1 ASP B, 200072 THR B 3.93 191.74 -0.2 -0.37 13.72 82 149 200070 ASP B, 3 LEU B, 200072 THR B, 1 ASP B 3.97 192.64 -0.98 -0.43 25.3 83 150 200070 ASP B, 3 LEU B, 200072 THR B, 1 ASP B, 63 LYS A 3.97 192.85 -1.56 -0.42 30.14 81 151 3 LEU B, 200072 THR B, 1 ASP B, 63 LYS A 3.89 193.97 -1.08 -0.27 25.25 79 152 3 LEU B, 1 ASP B, 63 LYS A 3.82 195.73 -1.2 -0.1 33.11 70 153 3 LEU B, 1 ASP B, 63 LYS A, 97 THR B 3.7 197.58 -1.08 -0.27 25.25 79 154 3 LEU B, 63 LYS A, 97 THR B, 2 ILE B 3.65 197.99 -0.3 -0.21 13.67 77 155 3 LEU B, 63 LYS A, 97 THR B, 98 PHE B 3.59 198.35 -0.3 -0.21 13.67 77 156 3 LEU B, 63 LYS A, 97 THR B, 98 PHE B, 61 ASN A 3.51 199.06 -0.94 -0.32 11.61 84 157 63 LYS A, 97 THR B, 98 PHE B, 61 ASN A, 46 GLU A 3.57 199.22 -2.4 -0.78 21.56 90 158 97 THR B, 98 PHE B, 61 ASN A, 46 GLU A 3.69 199.42 -2.03 -0.87 14.58 96 159 63 LYS A, 97 THR B, 98 PHE B, 61 ASN A, 46 GLU A 4.18 199.86 -2.4 -0.78 21.56 90 160 3 LEU B, 63 LYS A, 98 PHE B, 61 ASN A, 46 GLU A 4.53 200.65 -1.5 -0.4 21.26 76 161 3 LEU B, 63 LYS A, 98 PHE B, 46 GLU A 4.91 201.69 -1 -0.3 25.73 67 162 3 LEU B, 63 LYS A, 46 GLU A, 64 ILE A 5.26 205.24 0.23 0.35 24.92 76 163 3 LEU B, 63 LYS A, 46 GLU A, 200020 SER B 5.37 206.05 -1.1 -0.35 25.3 80 164 63 LYS A, 46 GLU A, 64 ILE A, 200020 SER B 4.96 206.78 -0.93 -0.18 25.3 92 165 63 LYS A, 46 GLU A, 64 ILE A 2.89 211.29 -0.97 0.09 33.18 84 166 46 GLU A, 64 ILE A, 63 LYS A 3.06 212.05 0.2 -0.04 17.8 90 167 46 GLU A, 64 ILE A, 63 LYS A, 200018 ARG B 3.24 214.48 -0.98 -0.14 26.35 87 168 46 GLU A, 64 ILE A, 200018 ARG B 3.75 215.04 -1.17 0.08 34.01 87 169 46 GLU A, 64 ILE A, 200018 ARG B, 40 ARG A 3.51 218.12 -2 -0.04 38.51 86 170 46 GLU A, 200018 ARG B, 40 ARG A 4.17 220.76 -4.17 -0.66 51.3 81 171 46 GLU A, 64 ILE A, 40 ARG A 3.99 221.25 -1.17 0.08 34.01 87 172 46 GLU A, 40 ARG A 3.28 224.56 -4 -0.78 50.95 80 173 40 ARG A 2.35 230.64 -4.5 -0.42 52 83 174 40 ARG A, 88 SER A 2.21 231.5 -2.45 -0.61 27.69 83 175 40 ARG A, 88 SER A, 91 SER A 2.14 231.98 -1.77 -0.67 19.59 83 176 40 ARG A, 88 SER A, 91 SER A 2.15 232.24 -1.9 -0.73 19.02 100 177 40 ARG A, 88 SER A, 91 SER A, 41 PRO A 2.23 232.47 -1.83 -0.57 14.66 86 178 40 ARG A, 88 SER A, 91 SER A, 41 PRO A 2.39 233.14 -1.73 -0.53 15.09 70 179 40 ARG A, 88 SER A, 41 PRO A 2.87 235.49 -2.17 -0.44 18.99 70 180 40 ARG A, 41 PRO A, 88 SER A 4.09 237.69 -2.3 -0.49 18.42 86 181 41 PRO A, 88 SER A 5.08 238.95 -1.2 -0.53 1.63 87 182 91 SER A, 41 PRO A, 88 SER A 4.81 239.87 -1.07 -0.68 1.64 97 183 91 SER A, 41 PRO A, 88 SER A, 175 LEU A 4.67 240.24 0.15 -0.22 1.26 86 184 41 PRO A, 88 SER A, 175 LEU A, 180 TYR A 4.46 240.75 0.02 0.3 1.25 69 185 91 SER A, 88 SER A, 175 LEU A, 180 TYR A 4.41 240.93 0.23 0.08 1.27 84 186 91 SER A, 88 SER A, 180 TYR A 4.37 241.12 -0.97 -0.28 1.65 94 187 91 SER A, 88 SER A 4.38 241.32 -0.8 -0.97 1.67 117 188 91 SER A, 88 SER A, 180 TYR A 4.39 241.77 -0.97 -0.28 1.65 94 189 88 SER A, 91 SER A, 41 PRO A, 175 LEU A, 180 TYR A 4.41 242.47 -0.06 0.08 1.67 69 190 91 SER A, 41 PRO A, 175 LEU A, 180 TYR A 4.44 243.34 0.02 0.3 1.25 69 191 41 PRO A, 175 LEU A, 180 TYR A 4.45 246.95 0.3 0.72 1.11 54 192 41 PRO A, 175 LEU A, 180 TYR A, 173 ALA A 4.41 247.38 0.13 0.34 1.68 54 193 41 PRO A, 175 LEU A, 180 TYR A 4.36 248.03 0.3 0.72 1.11 54 194 41 PRO A, 175 LEU A, 180 TYR A, 173 ALA A 4.39 248.64 0.13 0.34 1.68 54 195 41 PRO A, 180 TYR A, 173 ALA A 3.75 250.8 -1.1 0.07 2.19 54 196 41 PRO A, 180 TYR A, 173 ALA A, 153 GLU A 3.28 253 -1.7 -0.23 14.12 61 197 41 PRO A, 173 ALA A, 153 GLU A, 172 PRO A 3.28 255.02 -1.78 -0.53 14.11 64 198 41 PRO A, 153 GLU A, 172 PRO A, 41 ASN B 3.44 255.56 -2.55 -0.52 14.11 74 199 41 PRO A, 153 GLU A, 172 PRO A, 41 ASN B 3.48 256.47 -2.55 -0.52 14.11 74 200 41 PRO A, 172 PRO A, 41 ASN B, 42 GLY A 3.55 257.44 -1.78 -0.44 2.48 73 201 41 PRO A, 172 PRO A, 41 ASN B, 42 GLY A 3.64 257.65 -1.78 -0.44 2.48 73 202 41 PRO A, 172 PRO A, 41 ASN B, 42 GLY A 3.66 257.9 -1.78 -0.44 2.48 73 203 172 PRO A, 41 ASN B, 42 GLY A, 165 ASP B 3.61 258.61 -2.25 -0.68 14.51 82 204 172 PRO A, 41 ASN B, 42 GLY A, 165 ASP B, 40 THR B 3.57 258.88 -1.94 -0.69 11.94 88 205 41 ASN B, 42 GLY A, 165 ASP B, 40 THR B 3.33 261.2 -2.03 -0.85 14.53 99 206 42 GLY A, 165 ASP B, 40 THR B 3.26 262.5 -1.53 -0.87 18.25 96 207 42 GLY A, 165 ASP B, 85 ASN B 3.26 262.63 -2.47 -0.87 18.82 95 208 42 GLY A, 165 ASP B, 40 THR B, 85 ASN B 3.24 262.85 -2.03 -0.85 14.53 99 209 165 ASP B, 40 THR B, 85 ASN B 2.94 263.25 -2.57 -0.86 18.25 99 210 42 GLY A, 165 ASP B, 40 THR B, 85 ASN B 2.85 263.63 -2.03 -0.85 14.53 99 211 42 GLY A, 165 ASP B, 85 ASN B 2.7 268.77 -2.47 -0.87 18.82 95 212 42 GLY A, 165 ASP B, 85 ASN B, 103 LYS B 3.58 269.77 -2.83 -0.76 26.49 87 213 42 GLY A, 165 ASP B, 103 LYS B, 10 ILE B 3.93 269.96 -0.83 -0.11 25.68 87 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
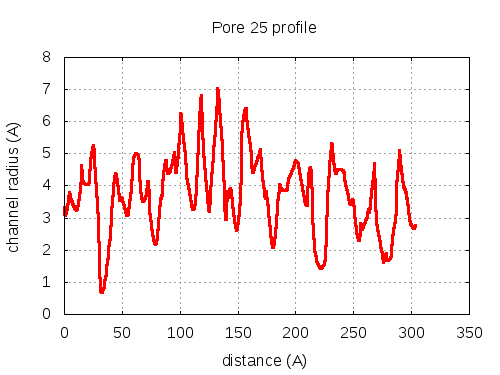
Unique lining residues set - all
200042 GLY A, 200040 THR B, 200085 ASN B, 200165 ASP B, 200041 ASN B, 200172 PRO A, 200041 PRO A, 200153 GLU A, 200173 ALA A, 200180 TYR A, 200175 LEU A, 200088 SER A, 200091 SER A, 200088 SER A, 200040 ARG A, 200091 SER A, 200043 HIS A, 200046 GLU A, 200064 ILE A, 100018 ARG B, 200063 LYS A, 200063 LYS A, 100020 SER B, 200003 LEU B, 200098 PHE B, 200061 ASN A, 200097 THR B, 200002 ILE B, 200001 ASP B, 100072 THR B, 100070 ASP B, 200001 ASP B, 200026 SER B, 100024 ARG B, 200026 SER B, 100069 THR B, 100026 SER B, 100027 GLN B, 100028 SER B, 100028 SER B, 200027 GLN B, 100027 GLN B, 200028 SER B, 200028 SER B, 27 GLN B, 28 SER B, 300027 GLN B, 300028 SER B, 200093 ARG B, 200082 TYR C, 100093 ARG B, 100079 GLY C, 200079 GLY C, 300093 ARG B, 100082 TYR C, 300079 GLY C, 300082 TYR C, 93 ARG B, 79 GLY C, 82 TYR C, 200058 GLN C, 200080 ASP C, 300028 SER B, 58 GLN C, 80 ASP C, 300026 SER B, 69 THR B, 27 GLN B, 26 SER B, 28 SER B, 24 ARG B, 70 ASP B, 300026 SER B, 300003 LEU B, 300001 ASP B, 72 THR B, 300001 ASP B, 300063 LYS A, 300097 THR B, 300002 ILE B, 300061 ASN A, 300098 PHE B, 300046 GLU A, 300064 ILE A, 20 SER B, 300063 LYS A, 18 ARG B, 300040 ARG A, 300088 SER A, 300091 SER A, 300041 PRO A, 300091 SER A, 300088 SER A, 300175 LEU A, 300180 TYR A, 300173 ALA A, 300153 GLU A, 300172 PRO A, 300041 ASN B, 300172 PRO A, 300182 LEU A, 300171 PHE A, 300170 THR A, 300155 VAL A, 300156 THR A, 300157 VAL A, 300040 THR B
Unique lining residues set - sidechains
200040 THR B, 200085 ASN B, 200165 ASP B, 200041 ASN B, 200172 PRO A, 200041 PRO A, 200153 GLU A, 200180 TYR A, 200175 LEU A, 200091 SER A, 200088 SER A, 200040 ARG A, 200043 HIS A, 200046 GLU A, 200064 ILE A, 100018 ARG B, 200063 LYS A, 100020 SER B, 200003 LEU B, 200061 ASN A, 200097 THR B, 200001 ASP B, 100072 THR B, 100070 ASP B, 200026 SER B, 100024 ARG B, 100069 THR B, 100028 SER B, 200027 GLN B, 100027 GLN B, 200028 SER B, 27 GLN B, 28 SER B, 300027 GLN B, 200093 ARG B, 200082 TYR C, 100093 ARG B, 300093 ARG B, 100082 TYR C, 300082 TYR C, 93 ARG B, 82 TYR C, 200058 GLN C, 300028 SER B, 58 GLN C, 69 THR B, 24 ARG B, 70 ASP B, 300026 SER B, 300003 LEU B, 72 THR B, 300001 ASP B, 300063 LYS A, 300097 THR B, 300061 ASN A, 300046 GLU A, 300064 ILE A, 20 SER B, 18 ARG B, 300040 ARG A, 300041 PRO A, 300091 SER A, 300088 SER A, 300175 LEU A, 300180 TYR A, 300153 GLU A, 300172 PRO A, 300041 ASN B, 300182 LEU A, 300170 THR A, 300156 THR A,
Physicochemical properties of lining side-chains
Charge: 3 (12-9)
Hydropathy: -1.5
Hydrophobicity: -0.41
Polarity: 15.09
Mutability: 87
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 200042 GLY A, 200040 THR B, 200085 ASN B, 200165 ASP B 3.23 0.13 -2.03 -0.85 14.53 99 2 200042 GLY A, 200040 THR B, 200165 ASP B 3.18 1.91 -1.53 -0.87 18.25 96 3 200042 GLY A, 200040 THR B, 200165 ASP B, 200041 ASN B 3.29 3.97 -2.03 -0.85 14.53 99 4 200042 GLY A, 200040 THR B, 200165 ASP B, 200041 ASN B, 200172 PRO A 3.6 4.19 -1.94 -0.69 11.94 88 5 200042 GLY A, 200165 ASP B, 200041 ASN B, 200172 PRO A 3.64 4.76 -2.25 -0.68 14.51 82 6 200042 GLY A, 200041 ASN B, 200172 PRO A, 200041 PRO A 3.68 5.1 -1.78 -0.44 2.48 73 7 200042 GLY A, 200041 ASN B, 200172 PRO A, 200041 PRO A 3.66 5.36 -1.78 -0.44 2.48 73 8 200042 GLY A, 200041 ASN B, 200172 PRO A, 200041 PRO A 3.58 6.36 -1.78 -0.44 2.48 73 9 200041 ASN B, 200172 PRO A, 200041 PRO A 3.56 6.67 -2.23 -0.32 2.18 73 10 200041 ASN B, 200172 PRO A, 200041 PRO A, 200153 GLU A 3.54 7.17 -2.55 -0.52 14.11 74 11 200041 ASN B, 200172 PRO A, 200041 PRO A, 200153 GLU A 3.53 7.73 -2.55 -0.52 14.11 74 12 200172 PRO A, 200041 PRO A, 200153 GLU A, 200173 ALA A 3.33 10.16 -1.78 -0.53 14.11 64 13 200041 PRO A, 200153 GLU A, 200173 ALA A, 200180 TYR A 3.29 11.93 -1.7 -0.23 14.12 61 14 200041 PRO A, 200173 ALA A, 200180 TYR A 3.46 14.3 -1.1 0.07 2.19 54 15 200041 PRO A, 200173 ALA A, 200180 TYR A, 200175 LEU A 4.3 14.63 0.13 0.34 1.68 54 16 200041 PRO A, 200180 TYR A, 200175 LEU A 4.35 15.29 0.3 0.72 1.11 54 17 200041 PRO A, 200173 ALA A, 200180 TYR A, 200175 LEU A 4.38 15.82 0.13 0.34 1.68 54 18 200041 PRO A, 200180 TYR A, 200175 LEU A 4.48 20.47 0.3 0.72 1.11 54 19 200041 PRO A, 200180 TYR A, 200175 LEU A, 200088 SER A, 200091 SER A 4.47 21.04 -0.06 0.08 1.67 69 20 200180 TYR A, 200175 LEU A, 200091 SER A, 200088 SER A 4.45 21.36 0.23 0.08 1.27 84 21 200180 TYR A, 200091 SER A, 200088 SER A 4.43 21.49 -0.97 -0.28 1.65 94 22 200091 SER A, 200088 SER A 4.41 21.56 -0.8 -0.97 1.67 117 23 200180 TYR A, 200091 SER A, 200088 SER A 4.4 21.89 -0.97 -0.28 1.65 94 24 200180 TYR A, 200175 LEU A, 200091 SER A, 200088 SER A 4.44 22.12 0.23 0.08 1.27 84 25 200041 PRO A, 200180 TYR A, 200175 LEU A, 200088 SER A 4.49 22.42 0.02 0.3 1.25 69 26 200041 PRO A, 200175 LEU A, 200091 SER A, 200088 SER A 4.58 23.24 0.15 -0.22 1.26 86 27 200041 PRO A, 200091 SER A, 200088 SER A 4.82 23.7 -1.07 -0.68 1.64 97 28 200041 PRO A, 200088 SER A 4.96 24.91 -1.2 -0.53 1.63 87 29 200041 PRO A, 200088 SER A, 200040 ARG A 4.13 27.67 -2.3 -0.49 18.42 86 30 200041 PRO A, 200088 SER A, 200040 ARG A 2.99 29.74 -2.17 -0.44 18.99 70 31 200041 PRO A, 200088 SER A, 200040 ARG A, 200091 SER A 2.73 30.06 -1.73 -0.53 15.09 70 32 200088 SER A, 200040 ARG A, 200091 SER A 1.91 31.5 -1.77 -0.67 19.59 83 33 200088 SER A, 200040 ARG A 1.79 32.45 -2.45 -0.61 27.69 83 34 200040 ARG A 1.73 39.19 -4.5 -0.42 52 83 35 200040 ARG A, 200043 HIS A 2.39 40.16 -3.85 -0.08 51.8 87 36 200040 ARG A, 200046 GLU A 2.74 43.32 -4 -0.78 50.95 80 37 200040 ARG A, 200046 GLU A, 200064 ILE A 4.21 43.94 -1.17 0.08 34.01 87 38 200040 ARG A, 200046 GLU A, 100018 ARG B 4.44 45.91 -4.17 -0.66 51.3 81 39 200040 ARG A, 200046 GLU A, 200064 ILE A, 100018 ARG B 3.49 49.41 -2 -0.04 38.51 86 40 200046 GLU A, 200064 ILE A, 100018 ARG B 3.62 49.97 -1.17 0.08 34.01 87 41 200046 GLU A, 200064 ILE A, 100018 ARG B, 200063 LYS A 2.78 53.21 -0.98 -0.14 26.35 87 42 200046 GLU A, 200064 ILE A, 200063 LYS A 2.73 58.2 -0.97 0.09 33.18 84 43 200046 GLU A, 200064 ILE A, 200063 LYS A, 100020 SER B 5.72 58.61 -0.93 -0.18 25.3 92 44 200046 GLU A, 200063 LYS A, 100020 SER B, 200003 LEU B 5.88 59.52 -1.1 -0.35 25.3 80 45 200046 GLU A, 200064 ILE A, 200063 LYS A, 200003 LEU B 5.15 62.58 0.23 0.35 24.92 76 46 200046 GLU A, 200063 LYS A, 200003 LEU B, 200098 PHE B 4.81 63.54 -1 -0.3 25.73 67 47 200046 GLU A, 200063 LYS A, 200003 LEU B, 200098 PHE B, 200061 ASN A 4.24 64.62 -1.5 -0.4 21.26 76 48 200046 GLU A, 200098 PHE B, 200061 ASN A, 200097 THR B 3.95 64.97 -2.03 -0.87 14.58 96 49 200046 GLU A, 200063 LYS A, 200098 PHE B, 200061 ASN A, 200097 THR B 3.96 65.17 -2.4 -0.78 21.56 90 50 200063 LYS A, 200003 LEU B, 200098 PHE B, 200061 ASN A, 200097 THR B 3.99 66 -0.94 -0.32 11.61 84 51 200063 LYS A, 200003 LEU B, 200098 PHE B, 200097 THR B 3.99 66.4 -0.3 -0.21 13.67 77 52 200063 LYS A, 200003 LEU B, 200097 THR B, 200002 ILE B 3.93 66.81 -0.3 -0.21 13.67 77 53 200063 LYS A, 200003 LEU B, 200097 THR B, 200001 ASP B 3.61 68.73 -1.08 -0.27 25.25 79 54 200063 LYS A, 200003 LEU B, 200001 ASP B 3.56 70.39 -1.2 -0.1 33.11 70 55 200063 LYS A, 200003 LEU B, 200001 ASP B, 100072 THR B 3.66 71.29 -1.08 -0.27 25.25 79 56 200063 LYS A, 200003 LEU B, 200001 ASP B, 100072 THR B, 100070 ASP B 3.93 71.48 -1.56 -0.42 30.14 81 57 200003 LEU B, 200001 ASP B, 100072 THR B, 100070 ASP B 3.91 72.45 -0.98 -0.43 25.3 83 58 200003 LEU B, 100070 ASP B, 200001 ASP B 3.02 74.78 -0.03 -0.23 17.74 70 59 200003 LEU B, 100070 ASP B 2.11 77.75 0.15 0.05 24.92 70 60 200003 LEU B, 100070 ASP B, 200026 SER B 2.08 80.08 -0.17 -0.29 17.17 85 61 200003 LEU B, 100070 ASP B, 200026 SER B, 100024 ARG B 2.44 82.16 -1.25 -0.32 25.88 85 62 100070 ASP B, 200026 SER B, 100024 ARG B 3.27 83.72 -2.93 -0.81 34.46 95 63 100070 ASP B, 100024 ARG B, 200026 SER B 3.71 84.85 -2.8 -0.75 35.03 84 64 100070 ASP B, 100024 ARG B, 200026 SER B, 100069 THR B 3.79 86.32 -2.28 -0.76 26.69 92 65 100024 ARG B, 200026 SER B, 100069 THR B 4.21 87.79 -1.87 -0.66 19.01 95 66 100024 ARG B, 200026 SER B, 100069 THR B, 100026 SER B 4.8 88.87 -1.5 -0.7 15.11 95 67 200026 SER B, 100069 THR B, 100026 SER B 4.41 91.84 -0.5 -0.79 2.81 107 68 200026 SER B, 100069 THR B, 100026 SER B, 100027 GLN B 4.38 92.72 -0.48 -0.79 2.95 107 69 200026 SER B, 100069 THR B, 100026 SER B, 100027 GLN B, 100028 SER B 4.42 94.43 -0.46 -0.79 3.04 107 70 200026 SER B, 100069 THR B, 100027 GLN B, 100028 SER B 4.77 94.62 -0.58 -0.84 2.52 112 71 200026 SER B, 100027 GLN B, 100028 SER B, 200027 GLN B 4.89 94.75 -1.28 -0.92 2.99 100 72 200026 SER B, 100069 THR B, 100028 SER B, 200027 GLN B 4.26 97.51 -1.35 -0.91 2.56 102 73 200026 SER B, 100028 SER B, 200027 GLN B 5 98.14 -1.57 -0.96 2.86 100 74 100027 GLN B, 100028 SER B, 200027 GLN B 5.37 98.77 -1.57 -0.96 2.86 100 75 100028 SER B, 200027 GLN B, 100027 GLN B 5.74 99.35 -2.6 -1.06 2.91 95 76 100028 SER B, 200027 GLN B, 100027 GLN B, 200028 SER B 6.08 99.8 -2.05 -0.99 3.03 95 77 100028 SER B, 200027 GLN B, 200028 SER B 6.36 100.1 -1.7 -1.01 2.29 106 78 27 GLN B, 28 SER B, 300027 GLN B, 300028 SER B 6.29 100.99 -2.05 -0.99 3.03 95 79 100028 SER B, 200027 GLN B, 200028 SER B 5.42 104.46 -1.7 -1.01 2.29 106 80 100028 SER B, 200027 GLN B, 200028 SER B, 200093 ARG B 5.06 104.93 -2.4 -0.87 14.72 100 81 200027 GLN B, 200028 SER B, 200093 ARG B 4.37 105.35 -2.93 -0.83 19.07 94 82 100028 SER B, 200027 GLN B, 200028 SER B, 200093 ARG B 4.08 105.76 -2.4 -0.87 14.72 100 83 100028 SER B, 200027 GLN B, 200028 SER B, 200093 ARG B 3.78 106.34 -2.3 -0.82 15.15 94 84 100028 SER B, 200027 GLN B, 200093 ARG B 2.91 111.43 -2.93 -0.83 19.07 94 85 100028 SER B, 200093 ARG B 3.27 112.12 -2.65 -0.7 26.84 100 86 100028 SER B, 200093 ARG B, 200082 TYR C 3.47 113.68 -2.2 -0.09 18.43 83 87 100028 SER B, 200093 ARG B, 200082 TYR C, 100093 ARG B 4.21 116.57 -2.78 -0.18 26.82 83 88 100028 SER B, 200093 ARG B, 200082 TYR C, 100093 ARG B, 100079 GLY C 6.09 117.33 -2.3 -0.3 22.13 83 89 200093 ARG B, 200082 TYR C, 100093 ARG B, 100079 GLY C, 200079 GLY C 6.46 117.93 -2.22 -0.27 22.47 72 90 100079 GLY C, 300093 ARG B, 100082 TYR C, 300079 GLY C, 300082 TYR C 6.67 118.16 -1.58 0.04 12.4 61 91 300093 ARG B, 300079 GLY C, 300082 TYR C, 93 ARG B, 79 GLY C 6.71 118.33 -2.22 -0.27 22.47 72 92 300093 ARG B, 100082 TYR C, 300079 GLY C, 300082 TYR C, 93 ARG B 6.45 118.76 -2.4 0.12 22.12 66 93 100093 ARG B, 300093 ARG B, 100082 TYR C, 300079 GLY C, 300082 TYR C 6.25 119.27 -2.4 0.12 22.12 66 94 200093 ARG B, 200082 TYR C, 100079 GLY C, 200079 GLY C, 82 TYR C 5.67 120.86 -1.58 0.04 12.4 61 95 200093 ARG B, 200082 TYR C, 200079 GLY C, 82 TYR C 5.3 121.78 -1.88 0.25 14.65 61 96 200093 ARG B, 200082 TYR C, 200079 GLY C, 200058 GLN C 3.23 124.9 -2.43 -0.3 15.13 72 97 200093 ARG B, 200079 GLY C, 200058 GLN C, 200080 ASP C 2.95 126.04 -2.2 -0.78 15.57 83 98 200093 ARG B, 200079 GLY C, 82 TYR C, 200058 GLN C 3.36 128.23 -2.43 -0.3 15.13 72 99 200093 ARG B, 200082 TYR C, 200079 GLY C, 82 TYR C, 200058 GLN C 4.91 129.1 -2.2 -0.02 12.43 66 100 200093 ARG B, 200082 TYR C, 200079 GLY C, 93 ARG B, 82 TYR C 5.5 130.68 -2.4 0.12 22.12 66 101 200093 ARG B, 200079 GLY C, 93 ARG B, 79 GLY C, 82 TYR C 6.54 131.27 -2.22 -0.27 22.47 72 102 200093 ARG B, 100093 ARG B, 300093 ARG B, 300079 GLY C, 93 ARG B 6.93 131.67 -3.68 -0.5 42.28 83 103 100093 ARG B, 100079 GLY C, 300093 ARG B, 100082 TYR C, 300079 GLY C 7.2 131.91 -2.22 -0.27 22.47 72 104 100093 ARG B, 100079 GLY C, 300093 ARG B, 100082 TYR C, 300028 SER B 7.37 132.14 -2.3 -0.3 22.13 83 105 100093 ARG B, 100079 GLY C, 300093 ARG B, 100082 TYR C, 300079 GLY C 7.34 132.45 -2.22 -0.27 22.47 72 106 200093 ARG B, 100093 ARG B, 100079 GLY C, 300093 ARG B, 100082 TYR C 7.14 132.98 -3.04 -0.19 32.2 74 107 200079 GLY C, 300082 TYR C, 93 ARG B, 79 GLY C, 82 TYR C 5.81 135.56 -1.58 0.04 12.4 61 108 93 ARG B, 79 GLY C, 82 TYR C, 58 GLN C 3.05 138.66 -2.43 -0.3 15.13 72 109 93 ARG B, 79 GLY C, 58 GLN C, 80 ASP C 3 139.34 -2.2 -0.78 15.57 83 110 300082 TYR C, 93 ARG B, 79 GLY C, 58 GLN C 3.2 140.4 -2.43 -0.3 15.13 72 111 300082 TYR C, 93 ARG B, 58 GLN C, 80 ASP C 3.97 140.58 -2.43 -0.3 15.13 72 112 300082 TYR C, 93 ARG B, 58 GLN C 3.75 141.54 -3.1 -0.14 19.05 72 113 28 SER B, 300082 TYR C, 93 ARG B, 58 GLN C 3.55 142.19 -2.53 -0.35 14.7 83 114 28 SER B, 93 ARG B, 58 GLN C 3.54 142.47 -2.93 -0.83 19.07 94 115 28 SER B, 300082 TYR C, 93 ARG B, 58 GLN C 3.74 143.6 -2.53 -0.35 14.7 83 116 28 SER B, 300093 ARG B, 300082 TYR C, 93 ARG B 4.04 143.97 -2.78 -0.18 26.82 83 117 28 SER B, 300093 ARG B, 300082 TYR C 3.78 145.63 -2.2 -0.09 18.43 83 118 28 SER B, 300093 ARG B 3.56 146.34 -2.65 -0.7 26.84 100 119 28 SER B, 300027 GLN B, 300093 ARG B 2.69 151.21 -2.93 -0.83 19.07 94 120 28 SER B, 300027 GLN B, 300028 SER B, 300093 ARG B 3.66 151.74 -2.3 -0.82 15.15 94 121 28 SER B, 300027 GLN B, 300093 ARG B, 300028 SER B 4.04 152.21 -2.4 -0.87 14.72 100 122 300027 GLN B, 300093 ARG B, 300028 SER B 4.73 152.54 -2.93 -0.83 19.07 94 123 28 SER B, 300027 GLN B, 300093 ARG B, 300028 SER B 5.22 153.03 -2.4 -0.87 14.72 100 124 28 SER B, 300027 GLN B, 300028 SER B 5.29 156.51 -1.7 -1.01 2.29 106 125 100028 SER B, 300027 GLN B, 300028 SER B 5.99 156.8 -1.7 -1.01 2.29 106 126 100028 SER B, 200027 GLN B, 100027 GLN B, 200028 SER B 6.15 157.71 -2.05 -0.99 3.03 95 127 27 GLN B, 28 SER B, 300027 GLN B, 300028 SER B 5.64 158.84 -2.05 -0.99 3.03 95 128 27 GLN B, 28 SER B, 300027 GLN B 5.33 159.48 -2.6 -1.06 2.91 95 129 28 SER B, 300027 GLN B, 300026 SER B 5.02 160.09 -1.57 -0.96 2.86 100 130 28 SER B, 300027 GLN B, 300026 SER B, 69 THR B 4.41 162.63 -1.35 -0.91 2.56 102 131 28 SER B, 300027 GLN B, 300026 SER B, 27 GLN B 4.74 163.03 -1.28 -0.92 2.99 100 132 300026 SER B, 69 THR B, 27 GLN B, 26 SER B, 28 SER B 4.57 165.91 -0.46 -0.79 3.04 107 133 300026 SER B, 69 THR B, 27 GLN B, 26 SER B 4.62 166.79 -0.48 -0.79 2.95 107 134 300026 SER B, 69 THR B, 26 SER B 4.7 168.89 -0.5 -0.79 2.81 107 135 300026 SER B, 69 THR B, 26 SER B, 24 ARG B 4.76 169.7 -1.5 -0.7 15.11 95 136 300026 SER B, 69 THR B, 24 ARG B 4.28 171.26 -1.87 -0.66 19.01 95 137 300026 SER B, 69 THR B, 24 ARG B, 70 ASP B 3.84 172.9 -2.28 -0.76 26.69 92 138 300026 SER B, 24 ARG B, 70 ASP B 3.73 173.65 -2.8 -0.75 35.03 84 139 24 ARG B, 70 ASP B, 300026 SER B 3.23 175.76 -2.93 -0.81 34.46 95 140 24 ARG B, 70 ASP B, 300026 SER B, 300003 LEU B 2.56 177.46 -1.25 -0.32 25.88 85 141 70 ASP B, 300026 SER B, 300003 LEU B 2.06 180.28 -0.17 -0.29 17.17 85 142 70 ASP B, 300003 LEU B 2.12 183.15 0.15 0.05 24.92 70 143 70 ASP B, 300003 LEU B, 300001 ASP B 3.1 184.93 -0.03 -0.23 17.74 70 144 70 ASP B, 300003 LEU B, 300001 ASP B, 72 THR B 3.93 185.06 -0.2 -0.37 13.72 82 145 70 ASP B, 300003 LEU B, 72 THR B, 300001 ASP B 3.99 185.92 -0.98 -0.43 25.3 83 146 70 ASP B, 300003 LEU B, 72 THR B, 300001 ASP B, 300063 LYS A 3.97 186.12 -1.56 -0.42 30.14 81 147 300003 LEU B, 72 THR B, 300001 ASP B, 300063 LYS A 3.68 187.7 -1.08 -0.27 25.25 79 148 300003 LEU B, 300001 ASP B, 300063 LYS A 3.65 189.39 -1.2 -0.1 33.11 70 149 300003 LEU B, 300001 ASP B, 300063 LYS A, 300097 THR B 3.73 190.7 -1.08 -0.27 25.25 79 150 300003 LEU B, 300063 LYS A, 300097 THR B, 300002 ILE B 3.87 191.11 -0.3 -0.21 13.67 77 151 300003 LEU B, 300063 LYS A, 300097 THR B, 300061 ASN A 3.88 191.48 -1.08 -0.2 13.67 84 152 300003 LEU B, 300063 LYS A, 300097 THR B, 300061 ASN A, 300098 PHE B 3.75 192.24 -0.94 -0.32 11.61 84 153 300063 LYS A, 300097 THR B, 300061 ASN A, 300098 PHE B, 300046 GLU A 3.71 192.43 -2.4 -0.78 21.56 90 154 300097 THR B, 300061 ASN A, 300098 PHE B, 300046 GLU A 3.7 192.81 -2.03 -0.87 14.58 96 155 300063 LYS A, 300097 THR B, 300061 ASN A, 300098 PHE B, 300046 GLU A 4.12 193.34 -2.4 -0.78 21.56 90 156 300003 LEU B, 300063 LYS A, 300061 ASN A, 300098 PHE B, 300046 GLU A 4.45 194.19 -1.5 -0.4 21.26 76 157 300003 LEU B, 300063 LYS A, 300098 PHE B, 300046 GLU A 4.84 195.23 -1 -0.3 25.73 67 158 300003 LEU B, 300063 LYS A, 300046 GLU A, 300064 ILE A 5.24 198.56 0.23 0.35 24.92 76 159 300003 LEU B, 300063 LYS A, 300046 GLU A, 20 SER B 5.79 199.37 -1.1 -0.35 25.3 80 160 300063 LYS A, 300046 GLU A, 300064 ILE A, 20 SER B 5.4 200.08 -0.93 -0.18 25.3 92 161 300063 LYS A, 300046 GLU A, 300064 ILE A 2.75 204.45 -0.97 0.09 33.18 84 162 300046 GLU A, 300064 ILE A, 300063 LYS A 2.84 205.19 0.2 -0.04 17.8 90 163 300046 GLU A, 300064 ILE A, 300063 LYS A, 18 ARG B 3.01 208.14 -0.98 -0.14 26.35 87 164 300046 GLU A, 300064 ILE A, 18 ARG B, 300040 ARG A 3.52 211.61 -2 -0.04 38.51 86 165 300046 GLU A, 18 ARG B, 300040 ARG A 4.24 214.12 -4.17 -0.66 51.3 81 166 300046 GLU A, 300064 ILE A, 300040 ARG A 4.07 214.62 -1.17 0.08 34.01 87 167 300046 GLU A, 300040 ARG A 3.37 217.88 -4 -0.78 50.95 80 168 300040 ARG A 2.37 223.83 -4.5 -0.42 52 83 169 300040 ARG A, 300088 SER A 2.21 224.71 -2.45 -0.61 27.69 83 170 300040 ARG A, 300088 SER A, 300091 SER A 2.09 225.51 -1.77 -0.67 19.59 83 171 300040 ARG A, 300088 SER A, 300041 PRO A, 300091 SER A 2.15 225.72 -1.83 -0.57 14.66 86 172 300040 ARG A, 300088 SER A, 300091 SER A, 300041 PRO A 2.29 226.76 -1.73 -0.53 15.09 70 173 300040 ARG A, 300088 SER A, 300041 PRO A 3 229.15 -2.17 -0.44 18.99 70 174 300040 ARG A, 300041 PRO A, 300088 SER A 4.3 231.13 -2.3 -0.49 18.42 86 175 300041 PRO A, 300088 SER A 4.98 232.4 -1.2 -0.53 1.63 87 176 300041 PRO A, 300091 SER A, 300088 SER A 4.66 233.27 -1.07 -0.68 1.64 97 177 300041 PRO A, 300091 SER A, 300088 SER A, 300175 LEU A 4.52 233.61 0.15 -0.22 1.26 86 178 300041 PRO A, 300088 SER A, 300175 LEU A, 300180 TYR A 4.43 233.88 0.02 0.3 1.25 69 179 300091 SER A, 300088 SER A, 300175 LEU A, 300180 TYR A 4.33 234.25 0.23 0.08 1.27 84 180 300091 SER A, 300088 SER A, 300180 TYR A 4.33 234.43 -0.97 -0.28 1.65 94 181 300091 SER A, 300088 SER A 4.35 234.64 -0.8 -0.97 1.67 117 182 300091 SER A, 300088 SER A, 300180 TYR A 4.38 235.08 -0.97 -0.28 1.65 94 183 300088 SER A, 300041 PRO A, 300091 SER A, 300175 LEU A, 300180 TYR A 4.4 235.76 -0.06 0.08 1.67 69 184 300041 PRO A, 300091 SER A, 300175 LEU A, 300180 TYR A 4.43 236.6 0.02 0.3 1.25 69 185 300041 PRO A, 300175 LEU A, 300180 TYR A 4.44 240.39 0.3 0.72 1.11 54 186 300041 PRO A, 300175 LEU A, 300180 TYR A, 300173 ALA A 4.38 240.65 0.13 0.34 1.68 54 187 300041 PRO A, 300175 LEU A, 300180 TYR A 4.36 241.29 0.3 0.72 1.11 54 188 300041 PRO A, 300175 LEU A, 300180 TYR A, 300173 ALA A 4.1 242.05 0.13 0.34 1.68 54 189 300041 PRO A, 300180 TYR A, 300173 ALA A 3.49 244.18 -1.1 0.07 2.19 54 190 300041 PRO A, 300180 TYR A, 300173 ALA A, 300153 GLU A 3.33 246.3 -1.7 -0.23 14.12 61 191 300041 PRO A, 300173 ALA A, 300153 GLU A, 300172 PRO A 3.41 248.31 -1.78 -0.53 14.11 64 192 300041 PRO A, 300153 GLU A, 300172 PRO A, 300041 ASN B 3.6 249.1 -2.55 -0.52 14.11 74 193 300041 PRO A, 300153 GLU A, 300172 PRO A, 300041 ASN B 3.34 249.71 -2.55 -0.52 14.11 74 194 300153 GLU A, 300172 PRO A, 300041 ASN B 3.15 250.09 -2.87 -0.67 18.29 79 195 300173 ALA A, 300153 GLU A, 300172 PRO A, 300041 ASN B 2.25 253.33 -2.25 -0.7 14.56 79 196 300173 ALA A, 300153 GLU A, 300041 ASN B, 300172 PRO A 2.26 254.24 -1.95 -0.88 15.01 90 197 300173 ALA A, 300153 GLU A, 300041 ASN B, 300172 PRO A, 300182 LEU A 2.36 254.52 -0.8 -0.47 12.03 78 198 300153 GLU A, 300041 ASN B, 300172 PRO A, 300182 LEU A, 300171 PHE A 2.44 254.74 -0.8 -0.47 12.03 78 199 300041 ASN B, 300172 PRO A, 300182 LEU A, 300171 PHE A 2.6 255.64 -0.13 -0.31 2.57 79 200 300041 ASN B, 300182 LEU A, 300171 PHE A 2.76 255.82 -0.03 -0.14 2.3 79 201 300041 ASN B, 300182 LEU A, 300170 THR A 2.78 256.11 -0.13 -0.13 1.72 88 202 300041 ASN B, 300182 LEU A, 300170 THR A, 300155 VAL A 2.72 257.79 -0.2 -0.3 2.14 88 203 300041 ASN B, 300170 THR A, 300155 VAL A 2.78 261.24 -1.53 -0.78 2.81 105 204 300041 ASN B, 300170 THR A, 300155 VAL A, 300156 THR A, 300157 VAL A 3.1 261.49 -1.14 -0.78 2.69 106 205 300041 ASN B, 300156 THR A, 300157 VAL A 3.15 261.74 -1.53 -0.78 2.81 105 206 300041 ASN B, 300170 THR A, 300156 THR A, 300157 VAL A 3.19 262.03 -1.33 -0.78 2.52 106 207 300041 ASN B, 300170 THR A, 300155 VAL A 3.17 262.33 -1.53 -0.78 2.81 105 208 300041 ASN B, 300170 THR A, 300157 VAL A 3.24 262.49 -1.53 -0.78 2.81 105 209 300041 ASN B, 300170 THR A, 300156 THR A, 300157 VAL A 3.18 262.91 -1.33 -0.78 2.52 106 210 300170 THR A, 300156 THR A, 300157 VAL A 3.23 263.1 -0.6 -0.78 2.23 107 211 300041 ASN B, 300170 THR A, 300156 THR A, 300157 VAL A 3.29 263.41 -1.33 -0.78 2.52 106 212 300041 ASN B, 300156 THR A, 300157 VAL A 3.38 266.24 -1.53 -0.78 2.81 105 213 300041 ASN B, 300156 THR A, 300157 VAL A, 300040 THR B 4.05 266.24 -1.25 -0.79 2.95 105 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
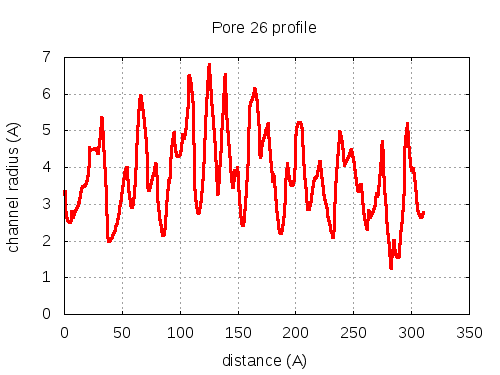
Unique lining residues set - all
100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B, 100155 VAL A, 100182 LEU A, 100171 PHE A, 100172 PRO A, 100153 GLU A, 100173 ALA A, 100172 PRO A, 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A, 100091 SER A, 100088 SER A, 100040 ARG A, 100091 SER A, 100043 HIS A, 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A, 100063 LYS A, 100003 LEU B, 300020 SER B, 100098 PHE B, 100061 ASN A, 100097 THR B, 100002 ILE B, 100001 ASP B, 300072 THR B, 300070 ASP B, 100001 ASP B, 100026 SER B, 300024 ARG B, 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B, 300028 SER B, 100027 GLN B, 300028 SER B, 100028 SER B, 300027 GLN B, 27 GLN B, 28 SER B, 200027 GLN B, 200028 SER B, 100028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 300079 GLY C, 100079 GLY C, 79 GLY C, 300082 TYR C, 93 ARG B, 200093 ARG B, 82 TYR C, 200079 GLY C, 200082 TYR C, 100058 GLN C, 100080 ASP C, 28 SER B, 200058 GLN C, 200080 ASP C, 200028 SER B, 26 SER B, 200069 THR B, 200027 GLN B, 200026 SER B, 200024 ARG B, 200070 ASP B, 26 SER B, 3 LEU B, 1 ASP B, 1 ASP B, 200072 THR B, 63 LYS A, 97 THR B, 2 ILE B, 98 PHE B, 61 ASN A, 46 GLU A, 64 ILE A, 200020 SER B, 63 LYS A, 200018 ARG B, 40 ARG A, 88 SER A, 91 SER A, 91 SER A, 41 PRO A, 88 SER A, 175 LEU A, 180 TYR A, 173 ALA A, 153 GLU A, 172 PRO A, 41 ASN B, 172 PRO A, 182 LEU A, 171 PHE A, 170 THR A, 155 VAL A, 156 THR A, 157 VAL A, 40 THR B
Unique lining residues set - sidechains
100156 THR A, 100170 THR A, 100041 ASN B, 100182 LEU A, 100153 GLU A, 100172 PRO A, 100041 PRO A, 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A, 100040 ARG A, 100043 HIS A, 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A, 100003 LEU B, 300020 SER B, 100061 ASN A, 100097 THR B, 100001 ASP B, 300072 THR B, 300070 ASP B, 100026 SER B, 300024 ARG B, 300069 THR B, 100027 GLN B, 300028 SER B, 300027 GLN B, 27 GLN B, 200027 GLN B, 200028 SER B, 100028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 300082 TYR C, 93 ARG B, 200093 ARG B, 82 TYR C, 200082 TYR C, 100058 GLN C, 28 SER B, 200058 GLN C, 200069 THR B, 200024 ARG B, 200070 ASP B, 26 SER B, 3 LEU B, 1 ASP B, 200072 THR B, 63 LYS A, 97 THR B, 61 ASN A, 46 GLU A, 64 ILE A, 200020 SER B, 200018 ARG B, 40 ARG A, 91 SER A, 41 PRO A, 88 SER A, 175 LEU A, 180 TYR A, 153 GLU A, 172 PRO A, 41 ASN B, 182 LEU A, 170 THR A, 156 THR A,
Physicochemical properties of lining side-chains
Charge: 4 (12-8)
Hydropathy: -1.5
Hydrophobicity: -0.42
Polarity: 14.62
Mutability: 86
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B 3.27 0.25 -1.33 -0.78 2.52 106 2 100157 VAL A, 100170 THR A, 100041 ASN B 3.21 0.43 -1.53 -0.78 2.81 105 3 100170 THR A, 100041 ASN B, 100155 VAL A 3.18 0.69 -1.53 -0.78 2.81 105 4 100157 VAL A, 100170 THR A, 100041 ASN B 3.19 0.84 -1.53 -0.78 2.81 105 5 100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B 3.2 0.99 -1.33 -0.78 2.52 106 6 100156 THR A, 100157 VAL A, 100041 ASN B 3.15 1.34 -1.53 -0.78 2.81 105 7 100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B, 100155 VAL A 3.11 1.79 -1.14 -0.78 2.69 106 8 100170 THR A, 100041 ASN B, 100155 VAL A 2.82 5.47 -1.53 -0.78 2.81 105 9 100170 THR A, 100041 ASN B, 100155 VAL A, 100182 LEU A 2.74 6.55 -0.2 -0.3 2.14 88 10 100170 THR A, 100041 ASN B, 100182 LEU A 2.78 6.87 -0.13 -0.13 1.72 88 11 100041 ASN B, 100182 LEU A, 100171 PHE A 2.74 7.22 -0.03 -0.14 2.3 79 12 100041 ASN B, 100182 LEU A, 100171 PHE A, 100172 PRO A 2.64 7.92 -0.13 -0.31 2.57 79 13 100041 ASN B, 100182 LEU A, 100171 PHE A, 100172 PRO A, 100153 GLU A 2.61 8.32 -0.8 -0.47 12.03 78 14 100041 ASN B, 100182 LEU A, 100172 PRO A, 100153 GLU A, 100173 ALA A 2.58 8.73 -0.8 -0.47 12.03 78 15 100041 ASN B, 100172 PRO A, 100153 GLU A, 100173 ALA A 2.52 9.83 -1.95 -0.88 15.01 90 16 100041 ASN B, 100153 GLU A, 100173 ALA A, 100172 PRO A 2.51 12.96 -2.25 -0.7 14.56 79 17 100041 ASN B, 100153 GLU A, 100172 PRO A 2.96 13.29 -2.87 -0.67 18.29 79 18 100041 ASN B, 100153 GLU A, 100172 PRO A, 100041 PRO A 3.1 13.82 -2.55 -0.52 14.11 74 19 100041 ASN B, 100153 GLU A, 100172 PRO A, 100041 PRO A 3.32 14.4 -2.55 -0.52 14.11 74 20 100153 GLU A, 100172 PRO A, 100041 PRO A 3.42 14.73 -2.23 -0.44 17.69 64 21 100153 GLU A, 100173 ALA A, 100172 PRO A, 100041 PRO A 3.27 16.95 -1.78 -0.53 14.11 64 22 100153 GLU A, 100173 ALA A, 100041 PRO A, 100180 TYR A 3.31 18.8 -1.7 -0.23 14.12 61 23 100173 ALA A, 100041 PRO A, 100180 TYR A 3.77 20.87 -1.1 0.07 2.19 54 24 100173 ALA A, 100041 PRO A, 100180 TYR A, 100175 LEU A 4.24 21.43 0.13 0.34 1.68 54 25 100041 PRO A, 100180 TYR A, 100175 LEU A 4.42 22.11 0.3 0.72 1.11 54 26 100173 ALA A, 100041 PRO A, 100180 TYR A, 100175 LEU A 4.33 22.52 0.13 0.34 1.68 54 27 100041 PRO A, 100180 TYR A, 100175 LEU A 4.03 27.28 0.3 0.72 1.11 54 28 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A, 100091 SER A 4.04 27.84 -0.06 0.08 1.67 69 29 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A 4.07 28.13 0.23 0.08 1.27 84 30 100180 TYR A, 100091 SER A, 100088 SER A 4.11 28.24 -0.97 -0.28 1.65 94 31 100091 SER A, 100088 SER A 4.17 28.33 -0.8 -0.97 1.67 117 32 100180 TYR A, 100091 SER A, 100088 SER A 4.25 28.49 -0.97 -0.28 1.65 94 33 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A 4.33 28.95 0.23 0.08 1.27 84 34 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A 4.55 29.29 0.02 0.3 1.25 69 35 100041 PRO A, 100175 LEU A, 100091 SER A, 100088 SER A 4.66 29.71 0.15 -0.22 1.26 86 36 100041 PRO A, 100091 SER A, 100088 SER A 4.77 30.66 -1.07 -0.68 1.64 97 37 100041 PRO A, 100088 SER A 4.95 31.82 -1.2 -0.53 1.63 87 38 100041 PRO A, 100088 SER A, 100040 ARG A 4.4 34.29 -2.3 -0.49 18.42 86 39 100041 PRO A, 100088 SER A, 100040 ARG A 3.25 36.45 -2.17 -0.44 18.99 70 40 100041 PRO A, 100088 SER A, 100040 ARG A, 100091 SER A 2.92 36.79 -1.73 -0.53 15.09 70 41 100088 SER A, 100040 ARG A, 100091 SER A 1.74 38.33 -1.77 -0.67 19.59 83 42 100088 SER A, 100040 ARG A 1.52 39.36 -2.45 -0.61 27.69 83 43 100040 ARG A 1.3 45.08 -4.5 -0.42 52 83 44 100040 ARG A, 100043 HIS A 1.71 46.25 -3.85 -0.08 51.8 87 45 100040 ARG A, 100046 GLU A 2.08 50.37 -4 -0.78 50.95 80 46 100040 ARG A, 100046 GLU A, 300018 ARG B 4.34 52.44 -4.17 -0.66 51.3 81 47 100040 ARG A, 100046 GLU A, 300018 ARG B, 100064 ILE A 3.45 56.14 -2 -0.04 38.51 86 48 100046 GLU A, 300018 ARG B, 100064 ILE A 3.67 56.72 -1.17 0.08 34.01 87 49 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A 2.9 60.09 -0.98 -0.14 26.35 87 50 100046 GLU A, 100064 ILE A, 100063 LYS A 2.78 65.16 -0.97 0.09 33.18 84 51 100046 GLU A, 100063 LYS A, 100003 LEU B, 300020 SER B 5.53 65.92 -1.1 -0.35 25.3 80 52 100046 GLU A, 100064 ILE A, 100063 LYS A, 100003 LEU B 5.33 69.97 0.23 0.35 24.92 76 53 100046 GLU A, 100063 LYS A, 100003 LEU B, 100098 PHE B 4.96 70.8 -1 -0.3 25.73 67 54 100046 GLU A, 100063 LYS A, 100003 LEU B, 100098 PHE B, 100061 ASN A 4.56 71.29 -1.5 -0.4 21.26 76 55 100046 GLU A, 100063 LYS A, 100098 PHE B, 100061 ASN A, 100097 THR B 4.2 71.47 -2.4 -0.78 21.56 90 56 100046 GLU A, 100098 PHE B, 100061 ASN A, 100097 THR B 3.68 71.65 -2.03 -0.87 14.58 96 57 100046 GLU A, 100063 LYS A, 100098 PHE B, 100061 ASN A, 100097 THR B 3.55 71.86 -2.4 -0.78 21.56 90 58 100063 LYS A, 100003 LEU B, 100098 PHE B, 100061 ASN A, 100097 THR B 3.48 72.71 -0.94 -0.32 11.61 84 59 100063 LYS A, 100003 LEU B, 100098 PHE B, 100097 THR B 3.57 73.12 -0.3 -0.21 13.67 77 60 100063 LYS A, 100003 LEU B, 100097 THR B, 100002 ILE B 3.63 73.55 -0.3 -0.21 13.67 77 61 100063 LYS A, 100003 LEU B, 100097 THR B, 100001 ASP B 3.69 75.54 -1.08 -0.27 25.25 79 62 100063 LYS A, 100003 LEU B, 100001 ASP B 3.85 77.23 -1.2 -0.1 33.11 70 63 100063 LYS A, 100003 LEU B, 100001 ASP B, 300072 THR B 3.93 78.08 -1.08 -0.27 25.25 79 64 100063 LYS A, 100003 LEU B, 100001 ASP B, 300072 THR B, 300070 ASP B 3.99 78.28 -1.56 -0.42 30.14 81 65 100003 LEU B, 100001 ASP B, 300072 THR B, 300070 ASP B 3.93 79.13 -0.98 -0.43 25.3 83 66 100003 LEU B, 300072 THR B, 300070 ASP B, 100001 ASP B 3.9 79.29 -0.2 -0.37 13.72 82 67 100003 LEU B, 300070 ASP B, 100001 ASP B 2.89 81.6 -0.03 -0.23 17.74 70 68 100003 LEU B, 300070 ASP B 2.25 84.18 0.15 0.05 24.92 70 69 100003 LEU B, 300070 ASP B, 100026 SER B 2.12 87.07 -0.17 -0.29 17.17 85 70 100003 LEU B, 300070 ASP B, 100026 SER B, 300024 ARG B 2.54 88.76 -1.25 -0.32 25.88 85 71 300070 ASP B, 100026 SER B, 300024 ARG B 3.25 90.43 -2.93 -0.81 34.46 95 72 300070 ASP B, 300024 ARG B, 100026 SER B 3.59 91.66 -2.8 -0.75 35.03 84 73 300070 ASP B, 300024 ARG B, 100026 SER B, 300069 THR B 3.8 93.23 -2.28 -0.76 26.69 92 74 300024 ARG B, 100026 SER B, 300069 THR B 4.5 94.64 -1.87 -0.66 19.01 95 75 300024 ARG B, 100026 SER B, 300069 THR B, 300026 SER B 4.83 95.43 -1.5 -0.7 15.11 95 76 100026 SER B, 300069 THR B, 300026 SER B 4.3 98.32 -0.5 -0.79 2.81 107 77 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B 4.33 99.24 -0.48 -0.79 2.95 107 78 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B, 300028 SER B 4.43 101.32 -0.46 -0.79 3.04 107 79 100026 SER B, 300027 GLN B, 100027 GLN B, 300028 SER B 5 101.45 -1.28 -0.92 2.99 100 80 100026 SER B, 300069 THR B, 100027 GLN B, 300028 SER B 4.2 104.41 -1.35 -0.91 2.56 102 81 100026 SER B, 100027 GLN B, 300028 SER B 5.15 105.07 -1.57 -0.96 2.86 100 82 300027 GLN B, 100027 GLN B, 300028 SER B 5.54 105.71 -1.57 -0.96 2.86 100 83 100027 GLN B, 300028 SER B, 100028 SER B, 300027 GLN B 5.91 106.67 -2.05 -0.99 3.03 95 84 27 GLN B, 28 SER B, 200027 GLN B, 200028 SER B 6.29 107.55 -2.05 -0.99 3.03 95 85 100027 GLN B, 300028 SER B, 100028 SER B 5.42 111.11 -1.7 -1.01 2.29 106 86 100027 GLN B, 300028 SER B, 100028 SER B, 100093 ARG B 5.2 111.64 -2.4 -0.87 14.72 100 87 100027 GLN B, 100028 SER B, 100093 ARG B 4.55 112.08 -2.93 -0.83 19.07 94 88 100027 GLN B, 300028 SER B, 100028 SER B, 100093 ARG B 4.21 112.5 -2.4 -0.87 14.72 100 89 100027 GLN B, 300028 SER B, 100028 SER B, 100093 ARG B 3.85 113.11 -2.3 -0.82 15.15 94 90 100027 GLN B, 300028 SER B, 100093 ARG B 2.67 118.4 -2.93 -0.83 19.07 94 91 300028 SER B, 100093 ARG B 3.32 119.09 -2.65 -0.7 26.84 100 92 300028 SER B, 100093 ARG B, 100082 TYR C 3.62 120.17 -2.2 -0.09 18.43 83 93 300028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B 4.22 123.16 -2.78 -0.18 26.82 83 94 300028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 300079 GLY C 5.97 123.96 -2.3 -0.3 22.13 83 95 100093 ARG B, 100082 TYR C, 300093 ARG B, 300079 GLY C, 100079 GLY C 6.39 124.6 -2.22 -0.27 22.47 72 96 300093 ARG B, 300079 GLY C, 100079 GLY C, 79 GLY C, 300082 TYR C 6.67 124.85 -1.4 -0.34 12.75 66 97 79 GLY C, 93 ARG B, 200093 ARG B, 82 TYR C, 200079 GLY C 6.71 125.03 -2.22 -0.27 22.47 72 98 79 GLY C, 300082 TYR C, 93 ARG B, 200093 ARG B, 82 TYR C 6.3 125.48 -2.4 0.12 22.12 66 99 300093 ARG B, 79 GLY C, 300082 TYR C, 93 ARG B, 82 TYR C 5.97 126 -2.4 0.12 22.12 66 100 100093 ARG B, 100082 TYR C, 300079 GLY C, 100079 GLY C, 200082 TYR C 5.13 127.66 -1.58 0.04 12.4 61 101 100093 ARG B, 100082 TYR C, 100079 GLY C, 200082 TYR C 4.68 128.61 -1.88 0.25 14.65 61 102 100093 ARG B, 100082 TYR C, 100079 GLY C, 100058 GLN C 3.32 131.7 -2.43 -0.3 15.13 72 103 100093 ARG B, 100079 GLY C, 100058 GLN C, 100080 ASP C 3.32 132.97 -2.2 -0.78 15.57 83 104 100093 ARG B, 100079 GLY C, 200082 TYR C, 100058 GLN C 3.8 135.37 -2.43 -0.3 15.13 72 105 100093 ARG B, 100082 TYR C, 100079 GLY C, 200082 TYR C, 100058 GLN C 4.91 136.26 -2.2 -0.02 12.43 66 106 100093 ARG B, 100082 TYR C, 100079 GLY C, 200093 ARG B, 200082 TYR C 5.33 137.08 -2.4 0.12 22.12 66 107 100093 ARG B, 100079 GLY C, 200093 ARG B, 200079 GLY C, 200082 TYR C 5.73 138.25 -2.22 -0.27 22.47 72 108 300093 ARG B, 300079 GLY C, 79 GLY C, 300082 TYR C, 93 ARG B 6.31 138.7 -2.22 -0.27 22.47 72 109 300093 ARG B, 300079 GLY C, 300082 TYR C, 93 ARG B, 28 SER B 6.25 139.07 -2.3 -0.3 22.13 83 110 100082 TYR C, 300093 ARG B, 300079 GLY C, 300082 TYR C, 93 ARG B 5.98 139.56 -2.4 0.12 22.12 66 111 100093 ARG B, 100079 GLY C, 200093 ARG B, 200079 GLY C, 200082 TYR C 5.63 140.3 -2.22 -0.27 22.47 72 112 100079 GLY C, 200093 ARG B, 82 TYR C, 200079 GLY C, 200082 TYR C 4.87 142.18 -1.58 0.04 12.4 61 113 200093 ARG B, 200079 GLY C, 200082 TYR C 4.52 143.12 -2.07 -0.04 19 66 114 200093 ARG B, 200079 GLY C, 200082 TYR C, 200058 GLN C 3.78 145.4 -2.43 -0.3 15.13 72 115 200093 ARG B, 200079 GLY C, 200058 GLN C, 200080 ASP C 3.78 146.1 -2.2 -0.78 15.57 83 116 200093 ARG B, 82 TYR C, 200079 GLY C, 200058 GLN C 3.7 147.18 -2.43 -0.3 15.13 72 117 200093 ARG B, 82 TYR C, 200058 GLN C, 200080 ASP C 3.66 147.37 -2.43 -0.3 15.13 72 118 200093 ARG B, 82 TYR C, 200058 GLN C 3.54 148.12 -3.1 -0.14 19.05 72 119 200093 ARG B, 82 TYR C, 200058 GLN C, 200028 SER B 3.51 148.38 -2.43 -0.3 15.13 72 120 200028 SER B, 200093 ARG B, 82 TYR C, 200058 GLN C 3.49 148.87 -2.53 -0.35 14.7 83 121 200028 SER B, 200093 ARG B, 200058 GLN C 3.54 149.15 -2.93 -0.83 19.07 94 122 200028 SER B, 200093 ARG B, 82 TYR C, 200058 GLN C 3.88 150.27 -2.53 -0.35 14.7 83 123 200028 SER B, 93 ARG B, 200093 ARG B, 82 TYR C 3.64 151.1 -2.78 -0.18 26.82 83 124 200028 SER B, 93 ARG B, 82 TYR C 3.12 152.37 -2.2 -0.09 18.43 83 125 200028 SER B, 93 ARG B 2.85 153.1 -2.65 -0.7 26.84 100 126 27 GLN B, 200028 SER B, 93 ARG B 2.44 158.12 -2.93 -0.83 19.07 94 127 27 GLN B, 28 SER B, 200028 SER B, 93 ARG B 4.33 158.59 -2.3 -0.82 15.15 94 128 27 GLN B, 200028 SER B, 93 ARG B, 28 SER B 4.68 158.85 -2.4 -0.87 14.72 100 129 27 GLN B, 93 ARG B, 28 SER B 4.94 159.32 -2.93 -0.83 19.07 94 130 27 GLN B, 200028 SER B, 93 ARG B, 28 SER B 5.1 160.02 -2.4 -0.87 14.72 100 131 27 GLN B, 200028 SER B, 28 SER B 5.1 163.46 -1.7 -1.01 2.29 106 132 100027 GLN B, 300028 SER B, 100028 SER B, 300027 GLN B 6.05 164.4 -2.05 -0.99 3.03 95 133 27 GLN B, 28 SER B, 200027 GLN B, 200028 SER B 5.53 165.57 -2.05 -0.99 3.03 95 134 27 GLN B, 200027 GLN B, 200028 SER B 5.23 166.23 -2.6 -1.06 2.91 95 135 27 GLN B, 200028 SER B, 26 SER B 4.94 166.86 -1.57 -0.96 2.86 100 136 27 GLN B, 200028 SER B, 26 SER B, 200069 THR B 4.47 169.39 -1.35 -0.91 2.56 102 137 27 GLN B, 200028 SER B, 26 SER B, 200027 GLN B 4.74 169.58 -1.28 -0.92 2.99 100 138 200028 SER B, 26 SER B, 200069 THR B, 200027 GLN B 4.65 169.95 -0.58 -0.84 2.52 112 139 200028 SER B, 26 SER B, 200069 THR B, 200027 GLN B, 200026 SER B 4.45 172.29 -0.46 -0.79 3.04 107 140 26 SER B, 200069 THR B, 200027 GLN B, 200026 SER B 4.47 173.22 -0.48 -0.79 2.95 107 141 26 SER B, 200069 THR B, 200026 SER B 4.55 175.55 -0.5 -0.79 2.81 107 142 26 SER B, 200069 THR B, 200026 SER B, 200024 ARG B 4.86 176.39 -1.5 -0.7 15.11 95 143 26 SER B, 200069 THR B, 200024 ARG B 4.28 178 -1.87 -0.66 19.01 95 144 26 SER B, 200069 THR B, 200024 ARG B, 200070 ASP B 3.75 179.65 -2.28 -0.76 26.69 92 145 26 SER B, 200024 ARG B, 200070 ASP B 3.72 180.32 -2.8 -0.75 35.03 84 146 200024 ARG B, 200070 ASP B, 26 SER B 3.22 182.34 -2.93 -0.81 34.46 95 147 200024 ARG B, 200070 ASP B, 26 SER B, 3 LEU B 2.49 184.08 -1.25 -0.32 25.88 85 148 200070 ASP B, 26 SER B, 3 LEU B 2.12 186.99 -0.17 -0.29 17.17 85 149 200070 ASP B, 3 LEU B 2.24 189.93 0.15 0.05 24.92 70 150 200070 ASP B, 3 LEU B, 1 ASP B 3.08 191.73 -0.03 -0.23 17.74 70 151 200070 ASP B, 3 LEU B, 1 ASP B, 200072 THR B 3.82 192.63 -0.98 -0.43 25.3 83 152 200070 ASP B, 3 LEU B, 1 ASP B, 200072 THR B, 63 LYS A 4.23 192.83 -1.56 -0.42 30.14 81 153 3 LEU B, 1 ASP B, 200072 THR B, 63 LYS A 4.12 193.94 -1.08 -0.27 25.25 79 154 3 LEU B, 1 ASP B, 63 LYS A 3.81 195.7 -1.2 -0.1 33.11 70 155 3 LEU B, 1 ASP B, 63 LYS A, 97 THR B 3.46 197.56 -1.08 -0.27 25.25 79 156 3 LEU B, 63 LYS A, 97 THR B, 2 ILE B 3.44 197.97 -0.3 -0.21 13.67 77 157 3 LEU B, 63 LYS A, 97 THR B, 98 PHE B 3.45 198.34 -0.3 -0.21 13.67 77 158 3 LEU B, 63 LYS A, 97 THR B, 98 PHE B, 61 ASN A 3.49 199.05 -0.94 -0.32 11.61 84 159 63 LYS A, 97 THR B, 98 PHE B, 61 ASN A, 46 GLU A 3.83 199.22 -2.4 -0.78 21.56 90 160 97 THR B, 98 PHE B, 61 ASN A, 46 GLU A 4.02 199.42 -2.03 -0.87 14.58 96 161 63 LYS A, 97 THR B, 98 PHE B, 61 ASN A, 46 GLU A 4.5 199.86 -2.4 -0.78 21.56 90 162 3 LEU B, 63 LYS A, 98 PHE B, 61 ASN A, 46 GLU A 4.73 200.65 -1.5 -0.4 21.26 76 163 3 LEU B, 63 LYS A, 98 PHE B, 46 GLU A 4.94 201.69 -1 -0.3 25.73 67 164 3 LEU B, 63 LYS A, 46 GLU A, 64 ILE A 5.05 205.24 0.23 0.35 24.92 76 165 3 LEU B, 63 LYS A, 46 GLU A, 200020 SER B 4.56 206.06 -1.1 -0.35 25.3 80 166 63 LYS A, 46 GLU A, 64 ILE A, 200020 SER B 4.25 206.79 -0.93 -0.18 25.3 92 167 63 LYS A, 46 GLU A, 64 ILE A 3.26 211.33 -0.97 0.09 33.18 84 168 46 GLU A, 64 ILE A, 63 LYS A 3.5 212.09 0.2 -0.04 17.8 90 169 46 GLU A, 64 ILE A, 63 LYS A, 200018 ARG B 3.62 214.52 -0.98 -0.14 26.35 87 170 46 GLU A, 64 ILE A, 200018 ARG B 3.77 215.08 -1.17 0.08 34.01 87 171 46 GLU A, 64 ILE A, 200018 ARG B, 40 ARG A 3.53 218.19 -2 -0.04 38.51 86 172 46 GLU A, 200018 ARG B, 40 ARG A 4.15 220.82 -4.17 -0.66 51.3 81 173 46 GLU A, 64 ILE A, 40 ARG A 4 221.34 -1.17 0.08 34.01 87 174 46 GLU A, 40 ARG A 3.59 223.31 -4 -0.78 50.95 80 175 40 ARG A 2.35 230.81 -4.5 -0.42 52 83 176 40 ARG A, 88 SER A 2.2 231.6 -2.45 -0.61 27.69 83 177 40 ARG A, 88 SER A, 91 SER A 2.12 232.04 -1.77 -0.67 19.59 83 178 40 ARG A, 88 SER A, 91 SER A 2.14 232.28 -1.9 -0.73 19.02 100 179 40 ARG A, 88 SER A, 91 SER A, 41 PRO A 2.24 232.53 -1.83 -0.57 14.66 86 180 40 ARG A, 88 SER A, 91 SER A, 41 PRO A 2.41 233.22 -1.73 -0.53 15.09 70 181 40 ARG A, 88 SER A, 41 PRO A 2.92 235.62 -2.17 -0.44 18.99 70 182 40 ARG A, 41 PRO A, 88 SER A 4.14 237.77 -2.3 -0.49 18.42 86 183 41 PRO A, 88 SER A 5.09 239.07 -1.2 -0.53 1.63 87 184 91 SER A, 41 PRO A, 88 SER A 4.85 239.97 -1.07 -0.68 1.64 97 185 91 SER A, 41 PRO A, 88 SER A, 175 LEU A 4.72 240.32 0.15 -0.22 1.26 86 186 41 PRO A, 88 SER A, 175 LEU A, 180 TYR A 4.59 240.59 0.02 0.3 1.25 69 187 91 SER A, 88 SER A, 175 LEU A, 180 TYR A 4.41 240.97 0.23 0.08 1.27 84 188 91 SER A, 88 SER A, 180 TYR A 4.3 241.16 -0.97 -0.28 1.65 94 189 91 SER A, 88 SER A 4.28 241.42 -0.8 -0.97 1.67 117 190 91 SER A, 88 SER A, 175 LEU A, 180 TYR A 4.27 241.94 0.23 0.08 1.27 84 191 88 SER A, 91 SER A, 41 PRO A, 175 LEU A, 180 TYR A 4.27 242.7 -0.06 0.08 1.67 69 192 41 PRO A, 175 LEU A, 180 TYR A 4.29 247.02 0.3 0.72 1.11 54 193 41 PRO A, 175 LEU A, 180 TYR A, 173 ALA A 4.46 247.3 0.13 0.34 1.68 54 194 41 PRO A, 175 LEU A, 180 TYR A 4.37 247.98 0.3 0.72 1.11 54 195 41 PRO A, 175 LEU A, 180 TYR A, 173 ALA A 4.45 248.79 0.13 0.34 1.68 54 196 41 PRO A, 180 TYR A, 173 ALA A 3.78 250.98 -1.1 0.07 2.19 54 197 41 PRO A, 180 TYR A, 173 ALA A, 153 GLU A 3.27 252.73 -1.7 -0.23 14.12 61 198 41 PRO A, 173 ALA A, 153 GLU A, 172 PRO A 3.26 254.87 -1.78 -0.53 14.11 64 199 41 PRO A, 153 GLU A, 172 PRO A 3.38 255.18 -2.23 -0.44 17.69 64 200 41 PRO A, 153 GLU A, 172 PRO A, 41 ASN B 3.26 255.71 -2.55 -0.52 14.11 74 201 41 PRO A, 153 GLU A, 172 PRO A, 41 ASN B 3.08 256.31 -2.55 -0.52 14.11 74 202 153 GLU A, 172 PRO A, 41 ASN B 2.89 257.12 -2.87 -0.67 18.29 79 203 173 ALA A, 153 GLU A, 172 PRO A, 41 ASN B 2.73 260 -2.25 -0.7 14.56 79 204 173 ALA A, 153 GLU A, 41 ASN B, 172 PRO A 2.73 260.95 -1.95 -0.88 15.01 90 205 173 ALA A, 153 GLU A, 41 ASN B, 172 PRO A, 182 LEU A 2.74 261.23 -0.8 -0.47 12.03 78 206 153 GLU A, 41 ASN B, 172 PRO A, 182 LEU A, 171 PHE A 2.72 261.45 -0.8 -0.47 12.03 78 207 41 ASN B, 172 PRO A, 182 LEU A, 171 PHE A 2.68 262.24 -0.13 -0.31 2.57 79 208 41 ASN B, 182 LEU A, 171 PHE A 2.77 262.6 -0.03 -0.14 2.3 79 209 41 ASN B, 182 LEU A, 170 THR A 2.73 262.86 -0.13 -0.13 1.72 88 210 41 ASN B, 182 LEU A, 170 THR A, 155 VAL A 2.58 264.38 -0.2 -0.3 2.14 88 211 41 ASN B, 170 THR A, 155 VAL A 2.82 267.94 -1.53 -0.78 2.81 105 212 41 ASN B, 170 THR A, 155 VAL A, 156 THR A, 157 VAL A 3.25 268.19 -1.14 -0.78 2.69 106 213 41 ASN B, 156 THR A, 157 VAL A 3.27 268.46 -1.53 -0.78 2.81 105 214 41 ASN B, 170 THR A, 156 THR A, 157 VAL A 3.24 268.62 -1.33 -0.78 2.52 106 215 41 ASN B, 170 THR A, 157 VAL A 3.22 268.75 -1.53 -0.78 2.81 105 216 41 ASN B, 170 THR A, 155 VAL A 3.18 268.97 -1.53 -0.78 2.81 105 217 41 ASN B, 170 THR A, 157 VAL A 3.24 269.14 -1.53 -0.78 2.81 105 218 41 ASN B, 170 THR A, 156 THR A, 157 VAL A 3.17 269.59 -1.33 -0.78 2.52 106 219 170 THR A, 156 THR A, 157 VAL A 3.22 269.75 -0.6 -0.78 2.23 107 220 41 ASN B, 170 THR A, 156 THR A, 157 VAL A 3.29 270.05 -1.33 -0.78 2.52 106 221 41 ASN B, 156 THR A, 157 VAL A 3.38 272.94 -1.53 -0.78 2.81 105 222 41 ASN B, 156 THR A, 157 VAL A, 40 THR B 4.05 272.94 -1.25 -0.79 2.95 105 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
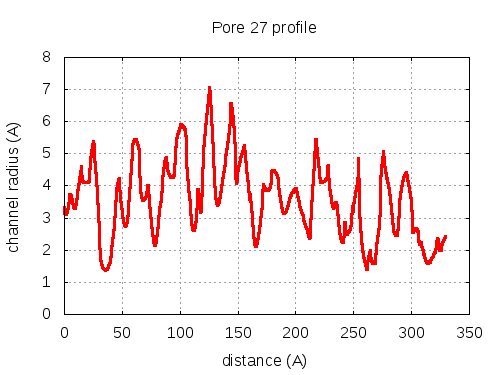
Unique lining residues set - all
100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B, 100155 VAL A, 100182 LEU A, 100171 PHE A, 100172 PRO A, 100153 GLU A, 100173 ALA A, 100172 PRO A, 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A, 100091 SER A, 100088 SER A, 100040 ARG A, 100091 SER A, 100043 HIS A, 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A, 100063 LYS A, 300020 SER B, 100003 LEU B, 100061 ASN A, 100098 PHE B, 100097 THR B, 100001 ASP B, 300072 THR B, 300070 ASP B, 100001 ASP B, 100026 SER B, 300024 ARG B, 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B, 300028 SER B, 300028 SER B, 100027 GLN B, 100028 SER B, 300027 GLN B, 27 GLN B, 28 SER B, 200027 GLN B, 200028 SER B, 100028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 300079 GLY C, 100079 GLY C, 93 ARG B, 79 GLY C, 82 TYR C, 200079 GLY C, 300082 TYR C, 200093 ARG B, 200082 TYR C, 100058 GLN C, 100080 ASP C, 200028 SER B, 28 SER B, 200026 SER B, 100069 THR B, 100027 GLN B, 100024 ARG B, 100070 ASP B, 200026 SER B, 200003 LEU B, 200001 ASP B, 100072 THR B, 200001 ASP B, 200063 LYS A, 200097 THR B, 200061 ASN A, 200098 PHE B, 200046 GLU A, 200064 ILE A, 100020 SER B, 200063 LYS A, 100018 ARG B, 200040 ARG A, 200088 SER A, 200091 SER A, 200091 SER A, 200041 PRO A, 200088 SER A, 200175 LEU A, 200180 TYR A, 200173 ALA A, 200153 GLU A, 200172 PRO A, 200041 ASN B, 200172 PRO A, 200171 PHE A, 200182 LEU A, 200170 THR A, 200155 VAL A, 200156 THR A, 200157 VAL A, 200040 THR B, 200168 VAL A, 200168 VAL A, 200169 LYS B, 200165 SER A, 200167 GLY A, 200169 HIS A, 200167 ASP B, 200166 SER A, 200166 SER A, 200170 ASP B, 200169 LYS B, 200140 MET A, 200138 ASN B, 200187 THR A, 200114 THR B, 200138 ASN A, 200139 SER A
Unique lining residues set - sidechains
100156 THR A, 100170 THR A, 100041 ASN B, 100182 LEU A, 100153 GLU A, 100172 PRO A, 100041 PRO A, 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A, 100040 ARG A, 100043 HIS A, 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A, 300020 SER B, 100003 LEU B, 100061 ASN A, 100097 THR B, 100001 ASP B, 300072 THR B, 300070 ASP B, 100026 SER B, 300024 ARG B, 300069 THR B, 300028 SER B, 100027 GLN B, 300027 GLN B, 27 GLN B, 200027 GLN B, 200028 SER B, 100028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 93 ARG B, 82 TYR C, 300082 TYR C, 200093 ARG B, 200082 TYR C, 100058 GLN C, 28 SER B, 100069 THR B, 100024 ARG B, 100070 ASP B, 200026 SER B, 200003 LEU B, 100072 THR B, 200001 ASP B, 200063 LYS A, 200097 THR B, 200061 ASN A, 200046 GLU A, 200064 ILE A, 100020 SER B, 100018 ARG B, 200040 ARG A, 200091 SER A, 200041 PRO A, 200088 SER A, 200175 LEU A, 200180 TYR A, 200153 GLU A, 200172 PRO A, 200041 ASN B, 200182 LEU A, 200170 THR A, 200156 THR A, 200168 VAL A, 200169 LYS B, 200169 HIS A, 200167 ASP B, 200166 SER A, 200170 ASP B, 200140 MET A, 200138 ASN B, 200187 THR A, 200114 THR B, 200138 ASN A, 200139 SER A
Physicochemical properties of lining side-chains
Charge: 3 (13-10)
Hydropathy: -1.4
Hydrophobicity: -0.44
Polarity: 14.63
Mutability: 88
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B 3.28 0.26 -1.33 -0.78 2.52 106 2 100157 VAL A, 100170 THR A, 100041 ASN B 3.3 0.47 -1.53 -0.78 2.81 105 3 100170 THR A, 100041 ASN B, 100155 VAL A 3.22 0.72 -1.53 -0.78 2.81 105 4 100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B 3.08 1.04 -1.33 -0.78 2.52 106 5 100156 THR A, 100157 VAL A, 100041 ASN B 2.9 1.25 -1.53 -0.78 2.81 105 6 100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B 2.81 1.74 -1.33 -0.78 2.52 106 7 100170 THR A, 100041 ASN B, 100155 VAL A 2.62 5.73 -1.53 -0.78 2.81 105 8 100170 THR A, 100041 ASN B, 100155 VAL A, 100182 LEU A 2.63 6.6 -0.2 -0.3 2.14 88 9 100170 THR A, 100041 ASN B, 100182 LEU A 2.75 7.01 -0.13 -0.13 1.72 88 10 100041 ASN B, 100182 LEU A, 100171 PHE A 2.74 7.2 -0.03 -0.14 2.3 79 11 100041 ASN B, 100182 LEU A, 100171 PHE A, 100172 PRO A 2.62 7.92 -0.13 -0.31 2.57 79 12 100041 ASN B, 100182 LEU A, 100171 PHE A, 100172 PRO A, 100153 GLU A 2.6 8.53 -0.8 -0.47 12.03 78 13 100041 ASN B, 100182 LEU A, 100172 PRO A, 100153 GLU A, 100173 ALA A 2.59 9.08 -0.8 -0.47 12.03 78 14 100041 ASN B, 100172 PRO A, 100153 GLU A, 100173 ALA A 2.58 9.74 -1.95 -0.88 15.01 90 15 100041 ASN B, 100153 GLU A, 100173 ALA A, 100172 PRO A 2.59 12.79 -2.25 -0.7 14.56 79 16 100041 ASN B, 100153 GLU A, 100172 PRO A 2.88 13.18 -2.87 -0.67 18.29 79 17 100041 ASN B, 100153 GLU A, 100172 PRO A, 100041 PRO A 2.99 13.8 -2.55 -0.52 14.11 74 18 100041 ASN B, 100153 GLU A, 100172 PRO A, 100041 PRO A 3.21 14.47 -2.55 -0.52 14.11 74 19 100153 GLU A, 100173 ALA A, 100172 PRO A, 100041 PRO A 3.36 16.98 -1.78 -0.53 14.11 64 20 100153 GLU A, 100173 ALA A, 100041 PRO A, 100180 TYR A 3.34 19.18 -1.7 -0.23 14.12 61 21 100173 ALA A, 100041 PRO A, 100180 TYR A 3.58 20.88 -1.1 0.07 2.19 54 22 100173 ALA A, 100041 PRO A, 100180 TYR A, 100175 LEU A 4.07 21.37 0.13 0.34 1.68 54 23 100041 PRO A, 100180 TYR A, 100175 LEU A 4.41 22.07 0.3 0.72 1.11 54 24 100173 ALA A, 100041 PRO A, 100180 TYR A, 100175 LEU A 4.2 22.59 0.13 0.34 1.68 54 25 100041 PRO A, 100180 TYR A, 100175 LEU A 4.04 27.23 0.3 0.72 1.11 54 26 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A, 100091 SER A 4.06 27.85 -0.06 0.08 1.67 69 27 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A 4.1 28.14 0.23 0.08 1.27 84 28 100091 SER A, 100088 SER A 4.15 28.22 -0.8 -0.97 1.67 117 29 100180 TYR A, 100091 SER A, 100088 SER A 4.23 28.58 -0.97 -0.28 1.65 94 30 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A 4.45 28.85 0.23 0.08 1.27 84 31 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A 4.59 29.23 0.02 0.3 1.25 69 32 100041 PRO A, 100175 LEU A, 100091 SER A, 100088 SER A 4.73 29.71 0.15 -0.22 1.26 86 33 100041 PRO A, 100091 SER A, 100088 SER A 4.88 30.79 -1.07 -0.68 1.64 97 34 100041 PRO A, 100088 SER A 5.14 31.68 -1.2 -0.53 1.63 87 35 100041 PRO A, 100088 SER A, 100040 ARG A 4.54 33.92 -2.3 -0.49 18.42 86 36 100041 PRO A, 100088 SER A, 100040 ARG A 2.77 36.43 -2.17 -0.44 18.99 70 37 100041 PRO A, 100088 SER A, 100040 ARG A, 100091 SER A 2.19 36.81 -1.73 -0.53 15.09 70 38 100088 SER A, 100040 ARG A, 100091 SER A 0.66 37.99 -1.77 -0.67 19.59 83 39 100088 SER A, 100040 ARG A 0.37 39.04 -2.45 -0.61 27.69 83 40 100040 ARG A 0.25 45.45 -4.5 -0.42 52 83 41 100040 ARG A, 100043 HIS A 1.84 46.67 -3.85 -0.08 51.8 87 42 100040 ARG A, 100043 HIS A, 100046 GLU A 2.52 47.67 -3.73 -0.43 51.17 83 43 100040 ARG A, 100046 GLU A 3.19 50.34 -4 -0.78 50.95 80 44 100040 ARG A, 100046 GLU A, 300018 ARG B 4.39 52.71 -4.17 -0.66 51.3 81 45 100040 ARG A, 100046 GLU A, 300018 ARG B, 100064 ILE A 3.43 55.99 -2 -0.04 38.51 86 46 100046 GLU A, 300018 ARG B, 100064 ILE A 3.62 56.65 -1.17 0.08 34.01 87 47 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A 3.54 59.68 -0.98 -0.14 26.35 87 48 100046 GLU A, 100064 ILE A, 100063 LYS A 3.48 60.55 0.2 -0.04 17.8 90 49 100046 GLU A, 100064 ILE A, 100063 LYS A 3.38 64.86 -0.97 0.09 33.18 84 50 100046 GLU A, 100064 ILE A, 100063 LYS A, 300020 SER B 3.84 65.31 -0.93 -0.18 25.3 92 51 100046 GLU A, 100063 LYS A, 300020 SER B, 100003 LEU B 4.02 65.73 -1.1 -0.35 25.3 80 52 100046 GLU A, 100064 ILE A, 100063 LYS A, 100003 LEU B 4.2 70.25 0.23 0.35 24.92 76 53 100046 GLU A, 100063 LYS A, 100003 LEU B, 100061 ASN A, 100098 PHE B 4.59 71.02 -1.5 -0.4 21.26 76 54 100046 GLU A, 100063 LYS A, 100061 ASN A, 100098 PHE B, 100097 THR B 4.58 71.35 -2.4 -0.78 21.56 90 55 100046 GLU A, 100061 ASN A, 100098 PHE B, 100097 THR B 4.46 71.54 -2.03 -0.87 14.58 96 56 100046 GLU A, 100063 LYS A, 100061 ASN A, 100098 PHE B, 100097 THR B 4.37 71.77 -2.4 -0.78 21.56 90 57 100063 LYS A, 100003 LEU B, 100061 ASN A, 100098 PHE B, 100097 THR B 4.03 72.81 -0.94 -0.32 11.61 84 58 100063 LYS A, 100003 LEU B, 100061 ASN A, 100097 THR B 3.91 73.29 -1.08 -0.2 13.67 84 59 100063 LYS A, 100003 LEU B, 100097 THR B, 100001 ASP B 3.59 75.57 -1.08 -0.27 25.25 79 60 100063 LYS A, 100003 LEU B, 100001 ASP B 3.58 76.89 -1.2 -0.1 33.11 70 61 100063 LYS A, 100003 LEU B, 100001 ASP B, 300072 THR B 3.65 77.95 -1.08 -0.27 25.25 79 62 100063 LYS A, 100003 LEU B, 100001 ASP B, 300072 THR B, 300070 ASP B 3.89 78.18 -1.56 -0.42 30.14 81 63 100003 LEU B, 100001 ASP B, 300072 THR B, 300070 ASP B 3.91 79.07 -0.98 -0.43 25.3 83 64 100003 LEU B, 300070 ASP B, 100001 ASP B 3.03 81.05 -0.03 -0.23 17.74 70 65 100003 LEU B, 300070 ASP B 2.25 83.94 0.15 0.05 24.92 70 66 100003 LEU B, 300070 ASP B, 100026 SER B 2.19 86.69 -0.17 -0.29 17.17 85 67 100003 LEU B, 300070 ASP B, 100026 SER B, 300024 ARG B 2.51 88.65 -1.25 -0.32 25.88 85 68 300070 ASP B, 100026 SER B, 300024 ARG B 3.25 90.31 -2.93 -0.81 34.46 95 69 300070 ASP B, 300024 ARG B, 100026 SER B 3.56 91.47 -2.8 -0.75 35.03 84 70 300070 ASP B, 300024 ARG B, 100026 SER B, 300069 THR B 3.88 93.27 -2.28 -0.76 26.69 92 71 300024 ARG B, 100026 SER B, 300069 THR B 4.62 94.38 -1.87 -0.66 19.01 95 72 300024 ARG B, 100026 SER B, 300069 THR B, 300026 SER B 5.01 95.41 -1.5 -0.7 15.11 95 73 100026 SER B, 300069 THR B, 300026 SER B 4.56 97.9 -0.5 -0.79 2.81 107 74 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B 4.44 98.96 -0.48 -0.79 2.95 107 75 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B, 300028 SER B 4.35 101.05 -0.46 -0.79 3.04 107 76 100026 SER B, 300069 THR B, 300027 GLN B, 300028 SER B 4.42 101.24 -0.58 -0.84 2.52 112 77 100026 SER B, 300027 GLN B, 300028 SER B, 100027 GLN B 4.5 101.42 -1.28 -0.92 2.99 100 78 100026 SER B, 300069 THR B, 300028 SER B, 100027 GLN B 4.58 104.15 -1.35 -0.91 2.56 102 79 100026 SER B, 300028 SER B, 100027 GLN B 5.15 104.9 -1.57 -0.96 2.86 100 80 300027 GLN B, 300028 SER B, 100027 GLN B 5.32 105.63 -1.57 -0.96 2.86 100 81 300028 SER B, 100027 GLN B, 100028 SER B, 300027 GLN B 5.53 106.64 -2.05 -0.99 3.03 95 82 27 GLN B, 28 SER B, 200027 GLN B, 200028 SER B 6 107.37 -2.05 -0.99 3.03 95 83 100027 GLN B, 200028 SER B, 100028 SER B 6.59 107.91 -1.7 -1.01 2.29 106 84 300028 SER B, 100027 GLN B, 100028 SER B 5.71 111.2 -1.7 -1.01 2.29 106 85 300028 SER B, 100027 GLN B, 100028 SER B, 100093 ARG B 5.02 111.5 -2.4 -0.87 14.72 100 86 100027 GLN B, 100028 SER B, 100093 ARG B 3.9 112.1 -2.93 -0.83 19.07 94 87 300028 SER B, 100027 GLN B, 100028 SER B, 100093 ARG B 3.56 112.71 -2.4 -0.87 14.72 100 88 300028 SER B, 100027 GLN B, 100028 SER B, 100093 ARG B 3.26 113.48 -2.3 -0.82 15.15 94 89 300028 SER B, 100027 GLN B, 100093 ARG B 2.75 117.95 -2.93 -0.83 19.07 94 90 300028 SER B, 100093 ARG B 3.21 118.76 -2.65 -0.7 26.84 100 91 300028 SER B, 100093 ARG B, 100082 TYR C 3.47 120.02 -2.2 -0.09 18.43 83 92 300028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B 4.1 122.94 -2.78 -0.18 26.82 83 93 300028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 300079 GLY C 6.04 123.86 -2.3 -0.3 22.13 83 94 100093 ARG B, 100082 TYR C, 300093 ARG B, 300079 GLY C, 100079 GLY C 6.5 124.56 -2.22 -0.27 22.47 72 95 93 ARG B, 79 GLY C, 82 TYR C, 200079 GLY C, 300082 TYR C 6.77 124.83 -1.58 0.04 12.4 61 96 93 ARG B, 79 GLY C, 82 TYR C, 300082 TYR C, 200093 ARG B 6.81 125.04 -2.4 0.12 22.12 66 97 300093 ARG B, 93 ARG B, 79 GLY C, 82 TYR C, 300082 TYR C 6.59 126.01 -2.4 0.12 22.12 66 98 100093 ARG B, 100082 TYR C, 300079 GLY C, 100079 GLY C, 200082 TYR C 6.06 128.08 -1.58 0.04 12.4 61 99 100093 ARG B, 100082 TYR C, 100079 GLY C, 200082 TYR C 5.69 129.17 -1.88 0.25 14.65 61 100 100093 ARG B, 100082 TYR C, 100079 GLY C, 100058 GLN C 4.03 131.3 -2.43 -0.3 15.13 72 101 100093 ARG B, 100079 GLY C, 100058 GLN C, 100080 ASP C 3.46 132.1 -2.2 -0.78 15.57 83 102 100093 ARG B, 100079 GLY C, 200082 TYR C, 100058 GLN C 3.26 133.15 -2.43 -0.3 15.13 72 103 100093 ARG B, 200082 TYR C, 100058 GLN C, 100080 ASP C 3.51 133.36 -2.43 -0.3 15.13 72 104 100093 ARG B, 200082 TYR C, 100058 GLN C 3.59 134.21 -3.1 -0.14 19.05 72 105 100028 SER B, 100093 ARG B, 200082 TYR C, 100058 GLN C 3.65 134.49 -2.43 -0.3 15.13 72 106 100028 SER B, 100093 ARG B, 200082 TYR C, 100058 GLN C 3.6 134.87 -2.53 -0.35 14.7 83 107 100028 SER B, 100093 ARG B, 100058 GLN C 3.57 135.21 -2.93 -0.83 19.07 94 108 100028 SER B, 100093 ARG B, 200082 TYR C, 100058 GLN C 3.68 136.58 -2.53 -0.35 14.7 83 109 100028 SER B, 100093 ARG B, 200093 ARG B, 200082 TYR C 3.91 137.07 -2.78 -0.18 26.82 83 110 100028 SER B, 200093 ARG B, 200082 TYR C 3.87 138.5 -2.2 -0.09 18.43 83 111 100028 SER B, 200093 ARG B 3.79 139.33 -2.65 -0.7 26.84 100 112 200027 GLN B, 100028 SER B, 200093 ARG B 3.36 144.29 -2.93 -0.83 19.07 94 113 200027 GLN B, 100028 SER B, 200093 ARG B, 200028 SER B 3.57 144.75 -2.3 -0.82 15.15 94 114 200027 GLN B, 200028 SER B, 100028 SER B, 200093 ARG B 3.74 144.95 -2.4 -0.87 14.72 100 115 200027 GLN B, 200028 SER B, 200093 ARG B 3.95 145.34 -2.93 -0.83 19.07 94 116 200027 GLN B, 200028 SER B, 100028 SER B, 200093 ARG B 4.77 146.18 -2.4 -0.87 14.72 100 117 200027 GLN B, 200028 SER B, 100028 SER B 5.38 149.47 -1.7 -1.01 2.29 106 118 300028 SER B, 300027 GLN B, 27 GLN B, 28 SER B 5.94 150.21 -2.05 -0.99 3.03 95 119 200027 GLN B, 200028 SER B, 100028 SER B 5.68 150.76 -1.7 -1.01 2.29 106 120 100027 GLN B, 200027 GLN B, 100028 SER B, 200028 SER B 5.4 151.45 -2.05 -0.99 3.03 95 121 100027 GLN B, 200027 GLN B, 100028 SER B 5.12 152.21 -2.6 -1.06 2.91 95 122 200027 GLN B, 100028 SER B, 200026 SER B 4.88 152.92 -1.57 -0.96 2.86 100 123 200027 GLN B, 100028 SER B, 200026 SER B, 100069 THR B 4.59 155.32 -1.35 -0.91 2.56 102 124 200027 GLN B, 100028 SER B, 200026 SER B, 100027 GLN B 4.82 155.8 -1.28 -0.92 2.99 100 125 100026 SER B, 100028 SER B, 200026 SER B, 100069 THR B, 100027 GLN B 4.45 158.34 -0.46 -0.79 3.04 107 126 100026 SER B, 200026 SER B, 100069 THR B, 100027 GLN B 4.36 159.4 -0.48 -0.79 2.95 107 127 100026 SER B, 200026 SER B, 100069 THR B 4.32 161.46 -0.5 -0.79 2.81 107 128 100026 SER B, 200026 SER B, 100069 THR B, 100024 ARG B 4.53 162.53 -1.5 -0.7 15.11 95 129 200026 SER B, 100069 THR B, 100024 ARG B 4.63 164.42 -1.87 -0.66 19.01 95 130 200026 SER B, 100069 THR B, 100024 ARG B, 100070 ASP B 4 165.69 -2.28 -0.76 26.69 92 131 200026 SER B, 100024 ARG B, 100070 ASP B 3.59 166.33 -2.8 -0.75 35.03 84 132 100024 ARG B, 100070 ASP B, 200026 SER B 3.31 168.28 -2.93 -0.81 34.46 95 133 100024 ARG B, 100070 ASP B, 200026 SER B, 200003 LEU B 2.48 170.28 -1.25 -0.32 25.88 85 134 100070 ASP B, 200026 SER B, 200003 LEU B 2.2 173.06 -0.17 -0.29 17.17 85 135 100070 ASP B, 200003 LEU B 2.34 175.86 0.15 0.05 24.92 70 136 100070 ASP B, 200003 LEU B, 200001 ASP B 2.98 177.66 -0.03 -0.23 17.74 70 137 100070 ASP B, 200003 LEU B, 200001 ASP B, 100072 THR B 3.98 177.79 -0.2 -0.37 13.72 82 138 100070 ASP B, 200003 LEU B, 100072 THR B, 200001 ASP B 4.02 178.81 -0.98 -0.43 25.3 83 139 200003 LEU B, 100072 THR B, 200001 ASP B, 200063 LYS A 3.79 180.12 -1.08 -0.27 25.25 79 140 200003 LEU B, 200001 ASP B, 200063 LYS A 3.57 182.11 -1.2 -0.1 33.11 70 141 200003 LEU B, 200001 ASP B, 200063 LYS A, 200097 THR B 3.5 183.63 -1.08 -0.27 25.25 79 142 200003 LEU B, 200063 LYS A, 200097 THR B, 200061 ASN A 3.55 184.09 -1.08 -0.2 13.67 84 143 200003 LEU B, 200063 LYS A, 200097 THR B, 200098 PHE B 3.62 184.47 -0.3 -0.21 13.67 77 144 200003 LEU B, 200063 LYS A, 200097 THR B, 200061 ASN A, 200098 PHE B 3.73 184.99 -0.94 -0.32 11.61 84 145 200063 LYS A, 200097 THR B, 200061 ASN A, 200098 PHE B, 200046 GLU A 4.07 185.19 -2.4 -0.78 21.56 90 146 200097 THR B, 200061 ASN A, 200098 PHE B, 200046 GLU A 4.31 185.46 -2.03 -0.87 14.58 96 147 200063 LYS A, 200097 THR B, 200061 ASN A, 200098 PHE B, 200046 GLU A 4.82 186.06 -2.4 -0.78 21.56 90 148 200003 LEU B, 200063 LYS A, 200061 ASN A, 200098 PHE B, 200046 GLU A 5.05 187.09 -1.5 -0.4 21.26 76 149 200003 LEU B, 200063 LYS A, 200098 PHE B, 200046 GLU A 5.23 188.35 -1 -0.3 25.73 67 150 200003 LEU B, 200063 LYS A, 200046 GLU A, 200064 ILE A 5.22 191.23 0.23 0.35 24.92 76 151 200003 LEU B, 200063 LYS A, 200046 GLU A, 100020 SER B 4.65 192.25 -1.1 -0.35 25.3 80 152 200063 LYS A, 200046 GLU A, 200064 ILE A 2.85 197.53 -0.97 0.09 33.18 84 153 200046 GLU A, 200064 ILE A, 200063 LYS A, 100018 ARG B 2.93 200.4 -0.98 -0.14 26.35 87 154 200046 GLU A, 200064 ILE A, 100018 ARG B 3.56 201.04 -1.17 0.08 34.01 87 155 200046 GLU A, 200064 ILE A, 100018 ARG B, 200040 ARG A 3.66 204.3 -2 -0.04 38.51 86 156 200046 GLU A, 100018 ARG B, 200040 ARG A 3.97 206.83 -4.17 -0.66 51.3 81 157 200046 GLU A, 200064 ILE A, 200040 ARG A 4.15 207.47 -1.17 0.08 34.01 87 158 200046 GLU A, 200040 ARG A 3.52 210.03 -4 -0.78 50.95 80 159 200040 ARG A 2.13 216.87 -4.5 -0.42 52 83 160 200040 ARG A, 200088 SER A 1.97 217.69 -2.45 -0.61 27.69 83 161 200040 ARG A, 200088 SER A, 200091 SER A 1.95 218.1 -1.77 -0.67 19.59 83 162 200040 ARG A, 200088 SER A, 200091 SER A 2.05 218.36 -1.9 -0.73 19.02 100 163 200040 ARG A, 200088 SER A, 200091 SER A, 200041 PRO A 2.25 219.1 -1.73 -0.53 15.09 70 164 200040 ARG A, 200088 SER A, 200041 PRO A 2.85 221.8 -2.17 -0.44 18.99 70 165 200040 ARG A, 200041 PRO A, 200088 SER A 4.21 223.8 -2.3 -0.49 18.42 86 166 200041 PRO A, 200088 SER A 4.94 225.32 -1.2 -0.53 1.63 87 167 200091 SER A, 200041 PRO A, 200088 SER A 4.82 225.83 -1.07 -0.68 1.64 97 168 200091 SER A, 200041 PRO A, 200088 SER A, 200175 LEU A 4.67 226.25 0.15 -0.22 1.26 86 169 200041 PRO A, 200088 SER A, 200175 LEU A, 200180 TYR A 4.52 226.56 0.02 0.3 1.25 69 170 200091 SER A, 200088 SER A, 200175 LEU A, 200180 TYR A 4.38 226.79 0.23 0.08 1.27 84 171 200091 SER A, 200088 SER A, 200180 TYR A 4.17 227.05 -0.97 -0.28 1.65 94 172 200091 SER A, 200088 SER A 4.12 227.24 -0.8 -0.97 1.67 117 173 200091 SER A, 200088 SER A, 200180 TYR A 4.1 227.74 -0.97 -0.28 1.65 94 174 200088 SER A, 200091 SER A, 200041 PRO A, 200175 LEU A, 200180 TYR A 4.11 228.57 -0.06 0.08 1.67 69 175 200091 SER A, 200041 PRO A, 200175 LEU A, 200180 TYR A 4.13 229.6 0.02 0.3 1.25 69 176 200041 PRO A, 200175 LEU A, 200180 TYR A 4.17 233.05 0.3 0.72 1.11 54 177 200041 PRO A, 200175 LEU A, 200180 TYR A, 200173 ALA A 4.29 233.24 0.13 0.34 1.68 54 178 200041 PRO A, 200175 LEU A, 200180 TYR A 4.32 233.93 0.3 0.72 1.11 54 179 200041 PRO A, 200175 LEU A, 200180 TYR A, 200173 ALA A 4.37 234.6 0.13 0.34 1.68 54 180 200041 PRO A, 200180 TYR A, 200173 ALA A 3.67 237.06 -1.1 0.07 2.19 54 181 200041 PRO A, 200180 TYR A, 200173 ALA A, 200153 GLU A 3.3 239.02 -1.7 -0.23 14.12 61 182 200041 PRO A, 200173 ALA A, 200153 GLU A, 200172 PRO A 3.31 240.98 -1.78 -0.53 14.11 64 183 200041 PRO A, 200153 GLU A, 200172 PRO A, 200041 ASN B 3.3 241.6 -2.55 -0.52 14.11 74 184 200041 PRO A, 200153 GLU A, 200172 PRO A, 200041 ASN B 3.04 242.28 -2.55 -0.52 14.11 74 185 200153 GLU A, 200172 PRO A, 200041 ASN B 2.89 242.72 -2.87 -0.67 18.29 79 186 200173 ALA A, 200153 GLU A, 200172 PRO A, 200041 ASN B 2.39 245.91 -2.25 -0.7 14.56 79 187 200173 ALA A, 200153 GLU A, 200041 ASN B, 200172 PRO A 2.39 246.96 -1.95 -0.88 15.01 90 188 200153 GLU A, 200041 ASN B, 200172 PRO A, 200171 PHE A, 200182 LEU A 2.46 247.35 -0.8 -0.47 12.03 78 189 200041 ASN B, 200172 PRO A, 200171 PHE A, 200182 LEU A 2.57 248.34 -0.13 -0.31 2.57 79 190 200041 ASN B, 200171 PHE A, 200182 LEU A 2.7 248.54 -0.03 -0.14 2.3 79 191 200041 ASN B, 200182 LEU A, 200170 THR A 2.67 248.71 -0.13 -0.13 1.72 88 192 200041 ASN B, 200182 LEU A, 200170 THR A, 200155 VAL A 2.61 250.56 -0.2 -0.3 2.14 88 193 200041 ASN B, 200170 THR A, 200155 VAL A 2.88 253.83 -1.53 -0.78 2.81 105 194 200041 ASN B, 200170 THR A, 200155 VAL A, 200156 THR A, 200157 VAL A 3.13 254.12 -1.14 -0.78 2.69 106 195 200041 ASN B, 200156 THR A, 200157 VAL A 3.16 254.45 -1.53 -0.78 2.81 105 196 200041 ASN B, 200170 THR A, 200156 THR A, 200157 VAL A 3.18 254.62 -1.33 -0.78 2.52 106 197 200041 ASN B, 200170 THR A, 200157 VAL A 3.18 254.71 -1.53 -0.78 2.81 105 198 200041 ASN B, 200170 THR A, 200155 VAL A 3.18 254.89 -1.53 -0.78 2.81 105 199 200041 ASN B, 200170 THR A, 200157 VAL A 3.23 255.04 -1.53 -0.78 2.81 105 200 200041 ASN B, 200170 THR A, 200156 THR A, 200157 VAL A 3.25 255.48 -1.33 -0.78 2.52 106 201 200041 ASN B, 200170 THR A, 200157 VAL A 3.29 258.52 -1.53 -0.78 2.81 105 202 200041 ASN B, 200170 THR A, 200157 VAL A, 200040 THR B 3.99 259.39 -1.25 -0.79 2.95 105 203 200041 ASN B, 200170 THR A, 200157 VAL A, 200040 THR B, 200168 VAL A 4.32 259.55 -1.08 -0.79 3.04 105 204 200170 THR A, 200157 VAL A, 200040 THR B, 200168 VAL A 4.41 259.75 0.68 -0.31 2.14 102 205 200170 THR A, 200157 VAL A, 200040 THR B, 200168 VAL A, 200169 LYS B 4.6 259.89 -1.16 -0.72 12.26 89 206 200170 THR A, 200157 VAL A, 200168 VAL A, 200169 LYS B 4.04 260.82 -0.2 -0.21 13.67 92 207 200157 VAL A, 200168 VAL A, 200169 LYS B, 200165 SER A 3.88 261.32 -0.13 -0.22 14.1 85 208 200168 VAL A, 200169 LYS B, 200165 SER A 2.83 265.36 -0.03 -0.03 17.67 85 209 200168 VAL A, 200169 LYS B, 200165 SER A 1.71 267.32 -1.57 -0.67 18.75 72 210 200168 VAL A, 200169 LYS B, 200165 SER A, 200167 GLY A 1.48 268.59 -1.28 -0.7 14.91 72 211 200168 VAL A, 200169 LYS B, 200167 GLY A 1.73 269.49 -1.57 -0.67 18.75 72 212 200168 VAL A, 200169 LYS B, 200167 GLY A, 200169 HIS A 1.94 269.77 -1.98 -0.44 26.97 81 213 200168 VAL A, 200169 LYS B, 200167 GLY A, 200169 HIS A 1.99 270.03 -1.98 -0.44 26.97 81 214 200168 VAL A, 200169 LYS B, 200167 GLY A, 200169 HIS A 1.94 270.05 -1.98 -0.44 26.97 81 215 200168 VAL A, 200169 LYS B, 200167 GLY A, 200169 HIS A 1.76 270.49 -1.98 -0.44 26.97 81 216 200169 LYS B, 200167 GLY A, 200169 HIS A, 200167 ASP B 1.71 270.98 -2.75 -0.5 38.55 83 217 200169 LYS B, 200167 GLY A, 200169 HIS A, 200167 ASP B 1.7 271.52 -2.75 -0.5 38.55 83 218 200169 LYS B, 200167 GLY A, 200167 ASP B 1.71 272.22 -2.6 -0.75 34.19 79 219 200169 LYS B, 200167 GLY A 1.73 273.7 -2.15 -0.61 26.44 72 220 200169 LYS B, 200167 GLY A, 200166 SER A 1.79 274.63 -1.57 -0.67 18.75 72 221 200169 LYS B, 200167 GLY A, 200166 SER A 1.89 278.26 -1.7 -0.73 18.18 94 222 200169 LYS B, 200167 GLY A, 200166 SER A, 200170 ASP B 2.95 279.53 -2.15 -0.81 26.06 91 223 200167 GLY A, 200166 SER A, 200170 ASP B, 200169 LYS B 4.37 279.99 -1.28 -0.9 14.53 101 224 200167 GLY A, 200166 SER A, 200170 ASP B 4.55 281.2 -1.57 -0.94 18.25 101 225 200167 GLY A, 200166 SER A, 200170 ASP B, 200140 MET A 4.96 281.95 -0.7 -0.45 14.05 98 226 200167 GLY A, 200170 ASP B, 200140 MET A 4.48 283.51 -0.67 -0.28 18.17 89 227 200167 GLY A, 200170 ASP B, 200140 MET A, 200138 ASN B 4.06 284.79 -1.38 -0.4 14.47 94 228 200170 ASP B, 200140 MET A, 200138 ASN B 3.91 286.45 -1.7 -0.27 18.17 94 229 200140 MET A, 200138 ASN B, 200187 THR A 3.64 288.17 -0.77 -0.18 2.16 101 230 200140 MET A, 200138 ASN B, 200187 THR A, 200114 THR B 3.06 290.02 -0.75 -0.33 2.03 102 231 200140 MET A, 200187 THR A, 200114 THR B 2.8 291.81 0.17 -0.18 1.58 102 232 200140 MET A, 200114 THR B 2.7 293.2 0.6 0.12 1.55 100 233 200140 MET A, 200114 THR B, 200138 ASN A, 200139 SER A 2.67 294.47 -0.78 -0.38 2.04 105 234 200140 MET A, 200114 THR B, 200138 ASN A 2.72 295.34 -0.77 -0.18 2.16 101 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
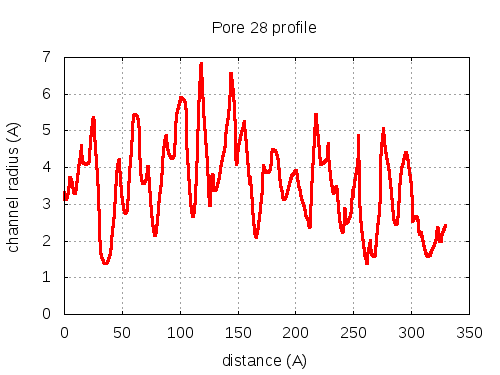
Unique lining residues set - all
100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B, 100155 VAL A, 100182 LEU A, 100171 PHE A, 100172 PRO A, 100153 GLU A, 100173 ALA A, 100172 PRO A, 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A, 100091 SER A, 100088 SER A, 100040 ARG A, 100091 SER A, 100043 HIS A, 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A, 100063 LYS A, 300020 SER B, 100003 LEU B, 100098 PHE B, 100061 ASN A, 100097 THR B, 100002 ILE B, 100001 ASP B, 300072 THR B, 300070 ASP B, 100001 ASP B, 100026 SER B, 300024 ARG B, 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B, 300028 SER B, 100027 GLN B, 300028 SER B, 300027 GLN B, 100028 SER B, 27 GLN B, 28 SER B, 200027 GLN B, 200028 SER B, 100028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 300079 GLY C, 100079 GLY C, 93 ARG B, 79 GLY C, 300082 TYR C, 200093 ARG B, 82 TYR C, 200079 GLY C, 200082 TYR C, 100058 GLN C, 100080 ASP C, 28 SER B, 200058 GLN C, 200080 ASP C, 200028 SER B, 200027 GLN B, 26 SER B, 200069 THR B, 200026 SER B, 200024 ARG B, 200070 ASP B, 26 SER B, 3 LEU B, 1 ASP B, 200072 THR B, 1 ASP B, 63 LYS A, 97 THR B, 61 ASN A, 98 PHE B, 46 GLU A, 64 ILE A, 200020 SER B, 63 LYS A, 200018 ARG B, 40 ARG A, 88 SER A, 91 SER A, 41 PRO A, 91 SER A, 88 SER A, 175 LEU A, 180 TYR A, 173 ALA A, 153 GLU A, 172 PRO A, 41 ASN B, 172 PRO A, 182 LEU A, 171 PHE A, 170 THR A, 155 VAL A, 156 THR A, 157 VAL A, 40 THR B, 168 VAL A, 168 VAL A, 169 LYS B, 165 SER A, 167 GLY A, 169 HIS A, 167 ASP B, 166 SER A, 166 SER A, 170 ASP B, 169 LYS B, 140 MET A, 138 ASN B, 187 THR A, 114 THR B, 138 ASN A, 139 SER A
Unique lining residues set - sidechains
100156 THR A, 100170 THR A, 100041 ASN B, 100182 LEU A, 100153 GLU A, 100172 PRO A, 100041 PRO A, 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A, 100040 ARG A, 100043 HIS A, 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A, 300020 SER B, 100003 LEU B, 100061 ASN A, 100097 THR B, 100001 ASP B, 300072 THR B, 300070 ASP B, 100026 SER B, 300024 ARG B, 300069 THR B, 100027 GLN B, 300028 SER B, 300027 GLN B, 27 GLN B, 200027 GLN B, 200028 SER B, 100028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 93 ARG B, 300082 TYR C, 200093 ARG B, 82 TYR C, 200082 TYR C, 100058 GLN C, 28 SER B, 200058 GLN C, 200069 THR B, 200024 ARG B, 200070 ASP B, 26 SER B, 3 LEU B, 200072 THR B, 1 ASP B, 63 LYS A, 97 THR B, 61 ASN A, 46 GLU A, 64 ILE A, 200020 SER B, 200018 ARG B, 40 ARG A, 41 PRO A, 91 SER A, 88 SER A, 175 LEU A, 180 TYR A, 153 GLU A, 172 PRO A, 41 ASN B, 182 LEU A, 170 THR A, 156 THR A, 168 VAL A, 169 LYS B, 169 HIS A, 167 ASP B, 166 SER A, 170 ASP B, 140 MET A, 138 ASN B, 187 THR A, 114 THR B, 138 ASN A, 139 SER A
Physicochemical properties of lining side-chains
Charge: 3 (13-10)
Hydropathy: -1.5
Hydrophobicity: -0.42
Polarity: 14.87
Mutability: 88
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B 3.27 0.25 -1.33 -0.78 2.52 106 2 100157 VAL A, 100170 THR A, 100041 ASN B 3.3 0.45 -1.53 -0.78 2.81 105 3 100170 THR A, 100041 ASN B, 100155 VAL A 3.29 0.67 -1.53 -0.78 2.81 105 4 100157 VAL A, 100170 THR A, 100041 ASN B 3.23 0.84 -1.53 -0.78 2.81 105 5 100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B 3.14 1 -1.33 -0.78 2.52 106 6 100156 THR A, 100157 VAL A, 100041 ASN B 2.82 1.51 -1.53 -0.78 2.81 105 7 100157 VAL A, 100170 THR A, 100041 ASN B, 100155 VAL A 2.71 2.15 -1.25 -0.79 2.95 105 8 100170 THR A, 100041 ASN B, 100155 VAL A 2.52 5.49 -1.53 -0.78 2.81 105 9 100170 THR A, 100041 ASN B, 100155 VAL A, 100182 LEU A 2.53 6.69 -0.2 -0.3 2.14 88 10 100170 THR A, 100041 ASN B, 100182 LEU A 2.79 6.89 -0.13 -0.13 1.72 88 11 100041 ASN B, 100182 LEU A, 100171 PHE A 2.75 7.27 -0.03 -0.14 2.3 79 12 100041 ASN B, 100182 LEU A, 100171 PHE A, 100172 PRO A 2.64 7.97 -0.13 -0.31 2.57 79 13 100041 ASN B, 100182 LEU A, 100171 PHE A, 100172 PRO A, 100153 GLU A 2.7 8.56 -0.8 -0.47 12.03 78 14 100041 ASN B, 100182 LEU A, 100172 PRO A, 100153 GLU A, 100173 ALA A 2.75 9.1 -0.8 -0.47 12.03 78 15 100041 ASN B, 100172 PRO A, 100153 GLU A, 100173 ALA A 2.79 9.74 -1.95 -0.88 15.01 90 16 100041 ASN B, 100153 GLU A, 100173 ALA A, 100172 PRO A 2.83 12.75 -2.25 -0.7 14.56 79 17 100041 ASN B, 100153 GLU A, 100172 PRO A 3.08 13.48 -2.87 -0.67 18.29 79 18 100041 ASN B, 100153 GLU A, 100172 PRO A, 100041 PRO A 3.2 13.77 -2.55 -0.52 14.11 74 19 100041 ASN B, 100153 GLU A, 100172 PRO A, 100041 PRO A 3.26 14.41 -2.55 -0.52 14.11 74 20 100153 GLU A, 100172 PRO A, 100041 PRO A 3.37 14.77 -2.23 -0.44 17.69 64 21 100153 GLU A, 100173 ALA A, 100172 PRO A, 100041 PRO A 3.41 16.83 -1.78 -0.53 14.11 64 22 100153 GLU A, 100173 ALA A, 100041 PRO A, 100180 TYR A 3.49 18.91 -1.7 -0.23 14.12 61 23 100173 ALA A, 100041 PRO A, 100180 TYR A 3.59 21.05 -1.1 0.07 2.19 54 24 100173 ALA A, 100041 PRO A, 100180 TYR A, 100175 LEU A 3.97 21.44 0.13 0.34 1.68 54 25 100041 PRO A, 100180 TYR A, 100175 LEU A 4.41 22.11 0.3 0.72 1.11 54 26 100173 ALA A, 100041 PRO A, 100180 TYR A, 100175 LEU A 4.47 22.68 0.13 0.34 1.68 54 27 100041 PRO A, 100180 TYR A, 100175 LEU A 4.48 27.26 0.3 0.72 1.11 54 28 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A, 100091 SER A 4.5 27.87 -0.06 0.08 1.67 69 29 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A 4.48 28.16 0.23 0.08 1.27 84 30 100091 SER A, 100088 SER A 4.44 28.24 -0.8 -0.97 1.67 117 31 100180 TYR A, 100091 SER A, 100088 SER A 4.39 28.59 -0.97 -0.28 1.65 94 32 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A 4.38 28.85 0.23 0.08 1.27 84 33 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A 4.4 29.2 0.02 0.3 1.25 69 34 100041 PRO A, 100175 LEU A, 100091 SER A, 100088 SER A 4.44 29.66 0.15 -0.22 1.26 86 35 100041 PRO A, 100091 SER A, 100088 SER A 4.53 30.71 -1.07 -0.68 1.64 97 36 100041 PRO A, 100088 SER A 4.82 31.62 -1.2 -0.53 1.63 87 37 100041 PRO A, 100088 SER A, 100040 ARG A 4.67 34.41 -2.3 -0.49 18.42 86 38 100041 PRO A, 100088 SER A, 100040 ARG A 3.42 36.27 -2.17 -0.44 18.99 70 39 100041 PRO A, 100088 SER A, 100040 ARG A, 100091 SER A 2.63 37.01 -1.73 -0.53 15.09 70 40 100088 SER A, 100040 ARG A, 100091 SER A 2.02 38.42 -1.77 -0.67 19.59 83 41 100088 SER A, 100040 ARG A 1.99 39.65 -2.45 -0.61 27.69 83 42 100040 ARG A 2.02 45.93 -4.5 -0.42 52 83 43 100040 ARG A, 100043 HIS A 2.57 47.03 -3.85 -0.08 51.8 87 44 100040 ARG A, 100046 GLU A 2.76 50.54 -4 -0.78 50.95 80 45 100040 ARG A, 100046 GLU A, 300018 ARG B 3.5 52.84 -4.17 -0.66 51.3 81 46 100040 ARG A, 100046 GLU A, 300018 ARG B, 100064 ILE A 3.39 56 -2 -0.04 38.51 86 47 100046 GLU A, 300018 ARG B, 100064 ILE A 3.22 56.64 -1.17 0.08 34.01 87 48 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A 2.91 59.59 -0.98 -0.14 26.35 87 49 100046 GLU A, 100064 ILE A, 100063 LYS A 3.08 60.45 0.2 -0.04 17.8 90 50 100046 GLU A, 100064 ILE A, 100063 LYS A 3.34 64.69 -0.97 0.09 33.18 84 51 100046 GLU A, 100064 ILE A, 100063 LYS A, 300020 SER B 5.75 65.24 -0.93 -0.18 25.3 92 52 100046 GLU A, 100063 LYS A, 300020 SER B, 100003 LEU B 5.94 66.28 -1.1 -0.35 25.3 80 53 100046 GLU A, 100064 ILE A, 100063 LYS A, 100003 LEU B 5.32 69.83 0.23 0.35 24.92 76 54 100046 GLU A, 100063 LYS A, 100003 LEU B, 100098 PHE B 4.97 70.76 -1 -0.3 25.73 67 55 100046 GLU A, 100063 LYS A, 100003 LEU B, 100098 PHE B, 100061 ASN A 4.59 71.26 -1.5 -0.4 21.26 76 56 100046 GLU A, 100063 LYS A, 100098 PHE B, 100061 ASN A, 100097 THR B 4.24 71.41 -2.4 -0.78 21.56 90 57 100046 GLU A, 100098 PHE B, 100061 ASN A, 100097 THR B 3.7 71.71 -2.03 -0.87 14.58 96 58 100046 GLU A, 100063 LYS A, 100098 PHE B, 100061 ASN A, 100097 THR B 3.53 71.94 -2.4 -0.78 21.56 90 59 100063 LYS A, 100003 LEU B, 100098 PHE B, 100061 ASN A, 100097 THR B 3.38 72.6 -0.94 -0.32 11.61 84 60 100063 LYS A, 100003 LEU B, 100098 PHE B, 100097 THR B 3.37 73.06 -0.3 -0.21 13.67 77 61 100063 LYS A, 100003 LEU B, 100097 THR B, 100002 ILE B 3.4 73.53 -0.3 -0.21 13.67 77 62 100063 LYS A, 100003 LEU B, 100097 THR B, 100001 ASP B 3.45 75.16 -1.08 -0.27 25.25 79 63 100063 LYS A, 100003 LEU B, 100001 ASP B 3.66 77.08 -1.2 -0.1 33.11 70 64 100063 LYS A, 100003 LEU B, 100001 ASP B, 300072 THR B 3.88 78.05 -1.08 -0.27 25.25 79 65 100063 LYS A, 100003 LEU B, 100001 ASP B, 300072 THR B, 300070 ASP B 4.05 78.27 -1.56 -0.42 30.14 81 66 100003 LEU B, 100001 ASP B, 300072 THR B, 300070 ASP B 4 79.12 -0.98 -0.43 25.3 83 67 100003 LEU B, 300070 ASP B, 100001 ASP B 2.9 81.46 -0.03 -0.23 17.74 70 68 100003 LEU B, 300070 ASP B 2.27 84.33 0.15 0.05 24.92 70 69 100003 LEU B, 300070 ASP B, 100026 SER B 2.15 87.01 -0.17 -0.29 17.17 85 70 100003 LEU B, 300070 ASP B, 100026 SER B, 300024 ARG B 2.49 88.9 -1.25 -0.32 25.88 85 71 300070 ASP B, 100026 SER B, 300024 ARG B 3.33 90.37 -2.93 -0.81 34.46 95 72 300070 ASP B, 300024 ARG B, 100026 SER B 3.59 91.59 -2.8 -0.75 35.03 84 73 300070 ASP B, 300024 ARG B, 100026 SER B, 300069 THR B 4.18 93.37 -2.28 -0.76 26.69 92 74 300024 ARG B, 100026 SER B, 300069 THR B 4.76 94.44 -1.87 -0.66 19.01 95 75 300024 ARG B, 100026 SER B, 300069 THR B, 300026 SER B 4.65 95.43 -1.5 -0.7 15.11 95 76 100026 SER B, 300069 THR B, 300026 SER B 4.29 98.84 -0.5 -0.79 2.81 107 77 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B, 300028 SER B 4.32 101.27 -0.46 -0.79 3.04 107 78 100026 SER B, 300027 GLN B, 100027 GLN B, 300028 SER B 4.69 101.42 -1.28 -0.92 2.99 100 79 100026 SER B, 300069 THR B, 100027 GLN B, 300028 SER B 4.79 104.59 -1.35 -0.91 2.56 102 80 100026 SER B, 100027 GLN B, 300028 SER B 5.05 105.33 -1.57 -0.96 2.86 100 81 100027 GLN B, 300028 SER B, 300027 GLN B 5.25 106 -2.6 -1.06 2.91 95 82 100027 GLN B, 300028 SER B, 300027 GLN B, 100028 SER B 5.49 106.51 -2.05 -0.99 3.03 95 83 27 GLN B, 28 SER B, 200027 GLN B, 200028 SER B 5.74 107.6 -2.05 -0.99 3.03 95 84 100027 GLN B, 300028 SER B, 100028 SER B 5.54 111.31 -1.7 -1.01 2.29 106 85 100027 GLN B, 300028 SER B, 100028 SER B, 100093 ARG B 4.86 111.59 -2.4 -0.87 14.72 100 86 100027 GLN B, 100028 SER B, 100093 ARG B 4.14 111.86 -2.93 -0.83 19.07 94 87 100027 GLN B, 300028 SER B, 100028 SER B, 100093 ARG B 3.49 112.84 -2.4 -0.87 14.72 100 88 100027 GLN B, 300028 SER B, 100028 SER B, 100093 ARG B 3.21 113.61 -2.3 -0.82 15.15 94 89 100027 GLN B, 300028 SER B, 100093 ARG B 2.75 117.97 -2.93 -0.83 19.07 94 90 300028 SER B, 100093 ARG B 3.18 118.77 -2.65 -0.7 26.84 100 91 300028 SER B, 100093 ARG B, 100082 TYR C 3.43 120.03 -2.2 -0.09 18.43 83 92 300028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B 4.04 123.36 -2.78 -0.18 26.82 83 93 300028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 300079 GLY C 6.29 123.81 -2.3 -0.3 22.13 83 94 100093 ARG B, 100082 TYR C, 300093 ARG B, 300079 GLY C, 100079 GLY C 6.5 124.53 -2.22 -0.27 22.47 72 95 300093 ARG B, 300079 GLY C, 93 ARG B, 79 GLY C, 300082 TYR C 6.77 124.8 -2.22 -0.27 22.47 72 96 93 ARG B, 79 GLY C, 200093 ARG B, 82 TYR C, 200079 GLY C 6.81 124.98 -2.22 -0.27 22.47 72 97 93 ARG B, 79 GLY C, 300082 TYR C, 200093 ARG B, 82 TYR C 6.7 125.22 -2.4 0.12 22.12 66 98 300093 ARG B, 93 ARG B, 79 GLY C, 300082 TYR C, 82 TYR C 6.53 125.68 -2.4 0.12 22.12 66 99 100093 ARG B, 100082 TYR C, 300093 ARG B, 300079 GLY C, 100079 GLY C 6.3 126.46 -2.22 -0.27 22.47 72 100 100093 ARG B, 100082 TYR C, 300079 GLY C, 100079 GLY C, 200082 TYR C 5.99 127.44 -1.58 0.04 12.4 61 101 100093 ARG B, 100082 TYR C, 100079 GLY C, 200082 TYR C 5.6 128.5 -1.88 0.25 14.65 61 102 100093 ARG B, 100082 TYR C, 100079 GLY C, 100058 GLN C 3.82 131.43 -2.43 -0.3 15.13 72 103 100093 ARG B, 100079 GLY C, 100058 GLN C 3.5 131.78 -2.8 -0.77 19.64 83 104 100093 ARG B, 100079 GLY C, 100058 GLN C, 100080 ASP C 3.29 132.75 -2.2 -0.78 15.57 83 105 100093 ARG B, 100079 GLY C, 200082 TYR C, 100058 GLN C 3.47 134.41 -2.43 -0.3 15.13 72 106 100093 ARG B, 100082 TYR C, 100079 GLY C, 200082 TYR C, 100058 GLN C 4.3 135.4 -2.2 -0.02 12.43 66 107 100093 ARG B, 100082 TYR C, 100079 GLY C, 200093 ARG B, 200082 TYR C 4.84 137.26 -2.4 0.12 22.12 66 108 100093 ARG B, 100079 GLY C, 200093 ARG B, 200079 GLY C, 200082 TYR C 5.89 137.93 -2.22 -0.27 22.47 72 109 300093 ARG B, 300079 GLY C, 93 ARG B, 79 GLY C, 300082 TYR C 6.28 138.57 -2.22 -0.27 22.47 72 110 300093 ARG B, 300079 GLY C, 93 ARG B, 300082 TYR C, 28 SER B 6.36 138.95 -2.3 -0.3 22.13 83 111 100082 TYR C, 300093 ARG B, 300079 GLY C, 93 ARG B, 300082 TYR C 6.04 139.5 -2.4 0.12 22.12 66 112 100093 ARG B, 100079 GLY C, 200093 ARG B, 200079 GLY C, 200082 TYR C 5.61 140.36 -2.22 -0.27 22.47 72 113 100079 GLY C, 200093 ARG B, 82 TYR C, 200079 GLY C, 200082 TYR C 5.14 141.4 -1.58 0.04 12.4 61 114 200093 ARG B, 82 TYR C, 200079 GLY C, 200082 TYR C 4.65 142.48 -1.88 0.25 14.65 61 115 200093 ARG B, 200079 GLY C, 200082 TYR C, 200058 GLN C 3.46 145.29 -2.43 -0.3 15.13 72 116 200093 ARG B, 200079 GLY C, 200058 GLN C, 200080 ASP C 3.49 146.05 -2.2 -0.78 15.57 83 117 200093 ARG B, 82 TYR C, 200079 GLY C, 200058 GLN C 3.68 147.03 -2.43 -0.3 15.13 72 118 200093 ARG B, 82 TYR C, 200058 GLN C, 200080 ASP C 3.89 147.24 -2.43 -0.3 15.13 72 119 200093 ARG B, 82 TYR C, 200058 GLN C 3.8 148.08 -3.1 -0.14 19.05 72 120 200093 ARG B, 82 TYR C, 200058 GLN C, 200028 SER B 3.78 148.36 -2.43 -0.3 15.13 72 121 200028 SER B, 200093 ARG B, 82 TYR C, 200058 GLN C 3.79 148.71 -2.53 -0.35 14.7 83 122 200028 SER B, 200093 ARG B, 200058 GLN C 3.89 149.04 -2.93 -0.83 19.07 94 123 200028 SER B, 200093 ARG B, 82 TYR C, 200058 GLN C 3.56 150.37 -2.53 -0.35 14.7 83 124 200028 SER B, 93 ARG B, 200093 ARG B, 82 TYR C 3.32 150.84 -2.78 -0.18 26.82 83 125 200028 SER B, 93 ARG B, 82 TYR C 2.61 153.02 -2.2 -0.09 18.43 83 126 27 GLN B, 200028 SER B, 93 ARG B 2.41 157.92 -2.93 -0.83 19.07 94 127 27 GLN B, 28 SER B, 200028 SER B, 93 ARG B 4.04 158.46 -2.3 -0.82 15.15 94 128 27 GLN B, 200028 SER B, 93 ARG B, 28 SER B 4.42 158.73 -2.4 -0.87 14.72 100 129 27 GLN B, 93 ARG B, 28 SER B 4.77 159.11 -2.93 -0.83 19.07 94 130 27 GLN B, 200028 SER B, 93 ARG B, 28 SER B 5.46 159.73 -2.4 -0.87 14.72 100 131 27 GLN B, 200028 SER B, 28 SER B 5.69 163.1 -1.7 -1.01 2.29 106 132 300028 SER B, 300027 GLN B, 28 SER B 6.02 163.42 -1.7 -1.01 2.29 106 133 100027 GLN B, 300028 SER B, 300027 GLN B, 100028 SER B 6.08 164.2 -2.05 -0.99 3.03 95 134 27 GLN B, 28 SER B, 200027 GLN B, 200028 SER B 6.01 165.49 -2.05 -0.99 3.03 95 135 27 GLN B, 200028 SER B, 200027 GLN B 5.86 166.23 -1.57 -0.96 2.86 100 136 27 GLN B, 200028 SER B, 26 SER B 5.67 166.9 -1.57 -0.96 2.86 100 137 27 GLN B, 200028 SER B, 26 SER B, 200069 THR B 4.28 169.16 -1.35 -0.91 2.56 102 138 27 GLN B, 200028 SER B, 200027 GLN B, 26 SER B 4.33 169.63 -1.28 -0.92 2.99 100 139 200028 SER B, 200027 GLN B, 26 SER B, 200069 THR B, 200026 SER B 4.5 172.11 -0.46 -0.79 3.04 107 140 200027 GLN B, 26 SER B, 200069 THR B, 200026 SER B 4.85 173.14 -0.48 -0.79 2.95 107 141 26 SER B, 200069 THR B, 200026 SER B 4.96 175.25 -0.5 -0.79 2.81 107 142 26 SER B, 200069 THR B, 200026 SER B, 200024 ARG B 4.9 176.25 -1.5 -0.7 15.11 95 143 26 SER B, 200069 THR B, 200024 ARG B 4.27 178.06 -1.87 -0.66 19.01 95 144 26 SER B, 200069 THR B, 200024 ARG B, 200070 ASP B 3.73 179.42 -2.28 -0.76 26.69 92 145 26 SER B, 200024 ARG B, 200070 ASP B 3.61 180.2 -2.8 -0.75 35.03 84 146 200024 ARG B, 200070 ASP B, 26 SER B 3.24 182.28 -2.93 -0.81 34.46 95 147 200024 ARG B, 200070 ASP B, 26 SER B, 3 LEU B 2.56 184.27 -1.25 -0.32 25.88 85 148 200070 ASP B, 26 SER B, 3 LEU B 2.21 186.99 -0.17 -0.29 17.17 85 149 200070 ASP B, 3 LEU B 2.24 189.72 0.15 0.05 24.92 70 150 200070 ASP B, 3 LEU B, 1 ASP B 2.94 191.46 -0.03 -0.23 17.74 70 151 200070 ASP B, 3 LEU B, 1 ASP B, 200072 THR B 3.92 191.61 -0.2 -0.37 13.72 82 152 200070 ASP B, 3 LEU B, 200072 THR B, 1 ASP B 3.95 192.55 -0.98 -0.43 25.3 83 153 200070 ASP B, 3 LEU B, 200072 THR B, 1 ASP B, 63 LYS A 3.95 192.8 -1.56 -0.42 30.14 81 154 3 LEU B, 200072 THR B, 1 ASP B, 63 LYS A 3.69 194.29 -1.08 -0.27 25.25 79 155 3 LEU B, 1 ASP B, 63 LYS A 3.56 195.62 -1.2 -0.1 33.11 70 156 3 LEU B, 1 ASP B, 63 LYS A, 97 THR B 3.51 197.65 -1.08 -0.27 25.25 79 157 3 LEU B, 63 LYS A, 97 THR B, 61 ASN A 3.66 198.08 -1.08 -0.2 13.67 84 158 3 LEU B, 63 LYS A, 97 THR B, 61 ASN A, 98 PHE B 3.76 198.9 -0.94 -0.32 11.61 84 159 63 LYS A, 97 THR B, 61 ASN A, 98 PHE B, 46 GLU A 4.27 199.07 -2.4 -0.78 21.56 90 160 97 THR B, 61 ASN A, 98 PHE B, 46 GLU A 4.49 199.37 -2.03 -0.87 14.58 96 161 63 LYS A, 97 THR B, 61 ASN A, 98 PHE B, 46 GLU A 4.91 200.05 -2.4 -0.78 21.56 90 162 3 LEU B, 63 LYS A, 61 ASN A, 98 PHE B, 46 GLU A 5.08 201.12 -1.5 -0.4 21.26 76 163 3 LEU B, 63 LYS A, 98 PHE B, 46 GLU A 5.2 202.37 -1 -0.3 25.73 67 164 3 LEU B, 63 LYS A, 46 GLU A, 64 ILE A 5.03 205.08 0.23 0.35 24.92 76 165 3 LEU B, 63 LYS A, 46 GLU A, 200020 SER B 4.47 206.11 -1.1 -0.35 25.3 80 166 63 LYS A, 46 GLU A, 64 ILE A 2.86 211.27 -0.97 0.09 33.18 84 167 46 GLU A, 64 ILE A, 63 LYS A, 200018 ARG B 2.93 214.74 -0.98 -0.14 26.35 87 168 46 GLU A, 64 ILE A, 200018 ARG B, 40 ARG A 3.62 217.88 -2 -0.04 38.51 86 169 46 GLU A, 200018 ARG B, 40 ARG A 3.85 220.43 -4.17 -0.66 51.3 81 170 46 GLU A, 64 ILE A, 40 ARG A 4.1 220.91 -1.17 0.08 34.01 87 171 46 GLU A, 40 ARG A 3.4 224.57 -4 -0.78 50.95 80 172 40 ARG A 2.24 231.01 -4.5 -0.42 52 83 173 40 ARG A, 88 SER A 2.11 231.68 -2.45 -0.61 27.69 83 174 40 ARG A, 88 SER A, 91 SER A 2.11 232 -1.77 -0.67 19.59 83 175 40 ARG A, 88 SER A, 41 PRO A, 91 SER A 2.23 232.26 -1.83 -0.57 14.66 86 176 40 ARG A, 88 SER A, 91 SER A, 41 PRO A 2.45 233.03 -1.73 -0.53 15.09 70 177 40 ARG A, 88 SER A, 41 PRO A 3.1 235.71 -2.17 -0.44 18.99 70 178 40 ARG A, 41 PRO A, 88 SER A 4.44 237.62 -2.3 -0.49 18.42 86 179 41 PRO A, 88 SER A 4.78 239.12 -1.2 -0.53 1.63 87 180 41 PRO A, 91 SER A, 88 SER A 4.67 239.62 -1.07 -0.68 1.64 97 181 41 PRO A, 91 SER A, 88 SER A, 175 LEU A 4.55 240.04 0.15 -0.22 1.26 86 182 41 PRO A, 88 SER A, 175 LEU A, 180 TYR A 4.43 240.35 0.02 0.3 1.25 69 183 91 SER A, 88 SER A, 175 LEU A, 180 TYR A 4.21 240.77 0.23 0.08 1.27 84 184 91 SER A, 88 SER A, 180 TYR A 4.13 240.86 -0.97 -0.28 1.65 94 185 91 SER A, 88 SER A 4.08 241.01 -0.8 -0.97 1.67 117 186 91 SER A, 88 SER A, 180 TYR A 4.07 241.42 -0.97 -0.28 1.65 94 187 41 PRO A, 88 SER A, 175 LEU A, 180 TYR A 4.09 242.16 0.02 0.3 1.25 69 188 88 SER A, 41 PRO A, 91 SER A, 175 LEU A, 180 TYR A 4.13 243.13 -0.06 0.08 1.67 69 189 41 PRO A, 175 LEU A, 180 TYR A 4.2 246.84 0.3 0.72 1.11 54 190 41 PRO A, 175 LEU A, 180 TYR A, 173 ALA A 4.47 247.16 0.13 0.34 1.68 54 191 41 PRO A, 175 LEU A, 180 TYR A 4.38 247.81 0.3 0.72 1.11 54 192 41 PRO A, 175 LEU A, 180 TYR A, 173 ALA A 4.34 248.6 0.13 0.34 1.68 54 193 41 PRO A, 180 TYR A, 173 ALA A 3.87 251.03 -1.1 0.07 2.19 54 194 41 PRO A, 180 TYR A, 173 ALA A, 153 GLU A 3.47 252.46 -1.7 -0.23 14.12 61 195 41 PRO A, 173 ALA A, 153 GLU A, 172 PRO A 3.35 254.83 -1.78 -0.53 14.11 64 196 41 PRO A, 153 GLU A, 172 PRO A, 41 ASN B 3.53 255.44 -2.55 -0.52 14.11 74 197 41 PRO A, 153 GLU A, 172 PRO A, 41 ASN B 3.46 256.1 -2.55 -0.52 14.11 74 198 153 GLU A, 172 PRO A, 41 ASN B 3.34 256.53 -2.87 -0.67 18.29 79 199 173 ALA A, 153 GLU A, 172 PRO A, 41 ASN B 2.51 259.63 -2.25 -0.7 14.56 79 200 173 ALA A, 153 GLU A, 41 ASN B, 172 PRO A 2.34 260.72 -1.95 -0.88 15.01 90 201 173 ALA A, 153 GLU A, 41 ASN B, 172 PRO A, 182 LEU A 2.32 261.02 -0.8 -0.47 12.03 78 202 153 GLU A, 41 ASN B, 172 PRO A, 182 LEU A, 171 PHE A 2.35 261.24 -0.8 -0.47 12.03 78 203 41 ASN B, 172 PRO A, 182 LEU A, 171 PHE A 2.47 262.08 -0.13 -0.31 2.57 79 204 41 ASN B, 182 LEU A, 171 PHE A 2.81 262.29 -0.03 -0.14 2.3 79 205 41 ASN B, 182 LEU A, 170 THR A 2.77 262.61 -0.13 -0.13 1.72 88 206 41 ASN B, 182 LEU A, 170 THR A, 155 VAL A 2.64 263.87 -0.2 -0.3 2.14 88 207 41 ASN B, 170 THR A, 155 VAL A 2.68 267.76 -1.53 -0.78 2.81 105 208 41 ASN B, 170 THR A, 155 VAL A, 156 THR A, 157 VAL A 3 267.99 -1.14 -0.78 2.69 106 209 41 ASN B, 156 THR A, 157 VAL A 3.07 268.3 -1.53 -0.78 2.81 105 210 41 ASN B, 170 THR A, 156 THR A, 157 VAL A 3.22 268.46 -1.33 -0.78 2.52 106 211 41 ASN B, 170 THR A, 157 VAL A 3.24 268.54 -1.53 -0.78 2.81 105 212 41 ASN B, 170 THR A, 155 VAL A 3.25 268.72 -1.53 -0.78 2.81 105 213 41 ASN B, 170 THR A, 157 VAL A 3.26 268.86 -1.53 -0.78 2.81 105 214 41 ASN B, 170 THR A, 156 THR A, 157 VAL A 3.22 269.27 -1.33 -0.78 2.52 106 215 41 ASN B, 170 THR A, 157 VAL A 3.21 272.57 -1.53 -0.78 2.81 105 216 41 ASN B, 170 THR A, 157 VAL A, 40 THR B 4.16 273.3 -1.25 -0.79 2.95 105 217 41 ASN B, 170 THR A, 157 VAL A, 40 THR B, 168 VAL A 4.58 273.43 -1.08 -0.79 3.04 105 218 170 THR A, 157 VAL A, 40 THR B, 168 VAL A 4.64 273.56 0.68 -0.31 2.14 102 219 170 THR A, 157 VAL A, 40 THR B, 168 VAL A, 169 LYS B 4.58 273.69 -1.16 -0.72 12.26 89 220 157 VAL A, 40 THR B, 168 VAL A, 169 LYS B 4.61 273.73 -0.13 -0.22 14.1 85 221 170 THR A, 157 VAL A, 168 VAL A, 169 LYS B 4.51 274.41 -0.2 -0.21 13.67 92 222 157 VAL A, 168 VAL A, 169 LYS B, 165 SER A 4 275.22 -0.13 -0.22 14.1 85 223 168 VAL A, 169 LYS B, 165 SER A 2.4 279.21 -0.03 -0.03 17.67 85 224 168 VAL A, 169 LYS B, 165 SER A 1.32 281.15 -1.57 -0.67 18.75 72 225 168 VAL A, 169 LYS B, 165 SER A, 167 GLY A 1.26 282.34 -1.28 -0.7 14.91 72 226 168 VAL A, 169 LYS B, 167 GLY A 1.65 283.55 -1.57 -0.67 18.75 72 227 168 VAL A, 169 LYS B, 167 GLY A, 169 HIS A 1.92 283.84 -1.98 -0.44 26.97 81 228 168 VAL A, 169 LYS B, 167 GLY A, 169 HIS A 2 283.95 -1.98 -0.44 26.97 81 229 168 VAL A, 169 LYS B, 167 GLY A, 169 HIS A 1.85 284.41 -1.98 -0.44 26.97 81 230 169 LYS B, 167 GLY A, 169 HIS A, 167 ASP B 1.72 285.07 -2.75 -0.5 38.55 83 231 169 LYS B, 167 GLY A, 167 ASP B 1.61 286.43 -2.6 -0.75 34.19 79 232 169 LYS B, 167 GLY A 1.58 287.17 -2.15 -0.61 26.44 72 233 169 LYS B, 167 GLY A, 166 SER A 1.56 288.55 -1.57 -0.67 18.75 72 234 169 LYS B, 167 GLY A, 166 SER A 1.64 288.7 -1.7 -0.73 18.18 94 235 169 LYS B, 166 SER A 1.82 288.91 -2.35 -0.69 25.59 94 236 169 LYS B, 167 GLY A, 166 SER A 1.94 292.19 -1.7 -0.73 18.18 94 237 169 LYS B, 167 GLY A, 166 SER A, 170 ASP B 3.14 293.4 -2.15 -0.81 26.06 91 238 167 GLY A, 166 SER A, 170 ASP B, 169 LYS B 4.64 293.86 -1.28 -0.9 14.53 101 239 167 GLY A, 166 SER A, 170 ASP B 4.8 295.04 -1.57 -0.94 18.25 101 240 167 GLY A, 166 SER A, 170 ASP B, 140 MET A 4.97 295.97 -0.7 -0.45 14.05 98 241 167 GLY A, 170 ASP B, 140 MET A 4.48 297.26 -0.67 -0.28 18.17 89 242 167 GLY A, 170 ASP B, 140 MET A, 138 ASN B 3.98 298.84 -1.38 -0.4 14.47 94 243 170 ASP B, 140 MET A, 138 ASN B 3.93 300.19 -1.7 -0.27 18.17 94 244 140 MET A, 138 ASN B, 187 THR A 3.65 302.14 -0.77 -0.18 2.16 101 245 140 MET A, 138 ASN B, 187 THR A, 114 THR B 3.02 303.95 -0.75 -0.33 2.03 102 246 140 MET A, 187 THR A, 114 THR B 2.75 305.78 0.17 -0.18 1.58 102 247 140 MET A, 114 THR B 2.69 306.5 0.6 0.12 1.55 100 248 140 MET A, 114 THR B, 138 ASN A 2.66 307.12 -0.77 -0.18 2.16 101 249 140 MET A, 114 THR B, 138 ASN A, 139 SER A 2.64 308.34 -0.78 -0.38 2.04 105 250 140 MET A, 114 THR B, 138 ASN A 2.7 309.19 -0.77 -0.18 2.16 101 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
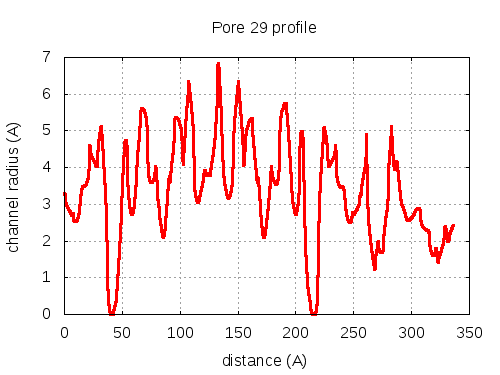
Unique lining residues set - all
100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B, 100155 VAL A, 100182 LEU A, 100171 PHE A, 100172 PRO A, 100153 GLU A, 100173 ALA A, 100172 PRO A, 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A, 100091 SER A, 100088 SER A, 100040 ARG A, 100091 SER A, 100043 HIS A, 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A, 100063 LYS A, 300020 SER B, 100003 LEU B, 100061 ASN A, 100098 PHE B, 100097 THR B, 100001 ASP B, 300072 THR B, 300070 ASP B, 100001 ASP B, 100026 SER B, 300024 ARG B, 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B, 300028 SER B, 100027 GLN B, 300028 SER B, 100028 SER B, 300027 GLN B, 27 GLN B, 28 SER B, 200027 GLN B, 200028 SER B, 100028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 300058 GLN C, 300079 GLY C, 300080 ASP C, 300082 TYR C, 79 GLY C, 93 ARG B, 200093 ARG B, 82 TYR C, 200079 GLY C, 200082 TYR C, 100079 GLY C, 28 SER B, 200028 SER B, 69 THR B, 27 GLN B, 26 SER B, 24 ARG B, 70 ASP B, 300026 SER B, 300003 LEU B, 300001 ASP B, 72 THR B, 300001 ASP B, 300063 LYS A, 300097 THR B, 300061 ASN A, 300098 PHE B, 300046 GLU A, 300064 ILE A, 20 SER B, 300063 LYS A, 18 ARG B, 300040 ARG A, 300088 SER A, 300091 SER A, 300041 PRO A, 300091 SER A, 300088 SER A, 300175 LEU A, 300180 TYR A, 300173 ALA A, 300153 GLU A, 300172 PRO A, 300041 ASN B, 300172 PRO A, 300182 LEU A, 300171 PHE A, 300170 THR A, 300155 VAL A, 300156 THR A, 300157 VAL A, 300040 THR B, 300168 VAL A, 300168 VAL A, 300169 LYS B, 300165 SER A, 300167 GLY A, 300169 HIS A, 300167 ASP B, 300166 SER A, 300166 SER A, 300170 ASP B, 300169 LYS B, 300140 MET A, 300138 ASN B, 300187 THR A, 300114 THR B, 300138 ASN A, 300139 SER A, 300113 PRO B, 300207 LYS B, 300115 VAL B, 300136 GLN A, 300116 SER B, 300140 MET A, 300135 ALA A, 300117 ILE B, 300116 SER B, 300117 ILE B, 300208 SER B, 300132 GLY A, 300209 PHE B, 300119 PRO B, 300133 SER A, 300210 ASN B, 300218 ARG A, 300211 ARG B, 300186 TYR B, 300211 ARG B, 300125 LEU B
Unique lining residues set - sidechains
100156 THR A, 100170 THR A, 100041 ASN B, 100182 LEU A, 100153 GLU A, 100172 PRO A, 100041 PRO A, 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A, 100040 ARG A, 100043 HIS A, 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A, 300020 SER B, 100003 LEU B, 100061 ASN A, 100097 THR B, 100001 ASP B, 300072 THR B, 300070 ASP B, 100026 SER B, 300024 ARG B, 300069 THR B, 100027 GLN B, 300028 SER B, 300027 GLN B, 27 GLN B, 200027 GLN B, 200028 SER B, 100028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 300058 GLN C, 300082 TYR C, 93 ARG B, 200093 ARG B, 82 TYR C, 200082 TYR C, 28 SER B, 69 THR B, 24 ARG B, 70 ASP B, 300026 SER B, 300003 LEU B, 72 THR B, 300001 ASP B, 300063 LYS A, 300097 THR B, 300061 ASN A, 300046 GLU A, 300064 ILE A, 20 SER B, 18 ARG B, 300040 ARG A, 300041 PRO A, 300091 SER A, 300088 SER A, 300175 LEU A, 300180 TYR A, 300153 GLU A, 300172 PRO A, 300041 ASN B, 300182 LEU A, 300170 THR A, 300156 THR A, 300168 VAL A, 300169 LYS B, 300169 HIS A, 300167 ASP B, 300166 SER A, 300170 ASP B, 300140 MET A, 300138 ASN B, 300187 THR A, 300114 THR B, 300138 ASN A, 300139 SER A, 300207 LYS B, 300116 SER B, 300135 ALA A, 300117 ILE B, 300209 PHE B, 300119 PRO B, 300218 ARG A, 300186 TYR B, 300211 ARG B, 300125 LEU B
Physicochemical properties of lining side-chains
Charge: 6 (16-10)
Hydropathy: -1.3
Hydrophobicity: -0.42
Polarity: 13.74
Mutability: 87
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B 3.27 0.28 -1.33 -0.78 2.52 106 2 100157 VAL A, 100170 THR A, 100041 ASN B 3.28 0.42 -1.53 -0.78 2.81 105 3 100170 THR A, 100041 ASN B, 100155 VAL A 3.26 0.62 -1.53 -0.78 2.81 105 4 100157 VAL A, 100170 THR A, 100041 ASN B 3.24 0.78 -1.53 -0.78 2.81 105 5 100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B 3.22 0.96 -1.33 -0.78 2.52 106 6 100156 THR A, 100157 VAL A, 100041 ASN B 3.08 1.43 -1.53 -0.78 2.81 105 7 100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B, 100155 VAL A 3.02 2.05 -1.14 -0.78 2.69 106 8 100170 THR A, 100041 ASN B, 100155 VAL A 2.79 5.5 -1.53 -0.78 2.81 105 9 100170 THR A, 100041 ASN B, 100155 VAL A, 100182 LEU A 2.71 6.58 -0.2 -0.3 2.14 88 10 100170 THR A, 100041 ASN B, 100182 LEU A 2.74 6.97 -0.13 -0.13 1.72 88 11 100041 ASN B, 100182 LEU A, 100171 PHE A 2.76 7.18 -0.03 -0.14 2.3 79 12 100041 ASN B, 100182 LEU A, 100171 PHE A, 100172 PRO A 2.61 7.94 -0.13 -0.31 2.57 79 13 100041 ASN B, 100182 LEU A, 100171 PHE A, 100172 PRO A, 100153 GLU A 2.56 8.51 -0.8 -0.47 12.03 78 14 100041 ASN B, 100182 LEU A, 100172 PRO A, 100153 GLU A, 100173 ALA A 2.54 9.06 -0.8 -0.47 12.03 78 15 100041 ASN B, 100172 PRO A, 100153 GLU A, 100173 ALA A 2.53 9.72 -1.95 -0.88 15.01 90 16 100041 ASN B, 100153 GLU A, 100173 ALA A, 100172 PRO A 2.53 12.83 -2.25 -0.7 14.56 79 17 100041 ASN B, 100153 GLU A, 100172 PRO A 2.81 13.23 -2.87 -0.67 18.29 79 18 100041 ASN B, 100153 GLU A, 100172 PRO A, 100041 PRO A 2.92 13.85 -2.55 -0.52 14.11 74 19 100041 ASN B, 100153 GLU A, 100172 PRO A, 100041 PRO A 3.13 14.55 -2.55 -0.52 14.11 74 20 100153 GLU A, 100173 ALA A, 100172 PRO A, 100041 PRO A 3.33 17.13 -1.78 -0.53 14.11 64 21 100153 GLU A, 100173 ALA A, 100041 PRO A, 100180 TYR A 3.5 18.75 -1.7 -0.23 14.12 61 22 100173 ALA A, 100041 PRO A, 100180 TYR A 3.55 21.03 -1.1 0.07 2.19 54 23 100173 ALA A, 100041 PRO A, 100180 TYR A, 100175 LEU A 3.87 21.44 0.13 0.34 1.68 54 24 100041 PRO A, 100180 TYR A, 100175 LEU A 4.32 22.03 0.3 0.72 1.11 54 25 100173 ALA A, 100041 PRO A, 100180 TYR A, 100175 LEU A 4.5 22.45 0.13 0.34 1.68 54 26 100041 PRO A, 100180 TYR A, 100175 LEU A 4.12 27.02 0.3 0.72 1.11 54 27 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A, 100091 SER A 4.06 27.74 -0.06 0.08 1.67 69 28 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A 4.03 28.11 0.23 0.08 1.27 84 29 100180 TYR A, 100091 SER A, 100088 SER A 4.03 28.56 -0.97 -0.28 1.65 94 30 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A 4.28 28.82 0.23 0.08 1.27 84 31 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A 4.42 29.19 0.02 0.3 1.25 69 32 100041 PRO A, 100175 LEU A, 100091 SER A, 100088 SER A 4.58 29.67 0.15 -0.22 1.26 86 33 100041 PRO A, 100091 SER A, 100088 SER A 4.73 30.77 -1.07 -0.68 1.64 97 34 100041 PRO A, 100088 SER A 4.99 31.68 -1.2 -0.53 1.63 87 35 100041 PRO A, 100088 SER A, 100040 ARG A 4.39 34 -2.3 -0.49 18.42 86 36 100041 PRO A, 100088 SER A, 100040 ARG A 2.7 36.51 -2.17 -0.44 18.99 70 37 100041 PRO A, 100088 SER A, 100040 ARG A, 100091 SER A 2.14 36.89 -1.73 -0.53 15.09 70 38 100088 SER A, 100040 ARG A, 100091 SER A 0.61 38.21 -1.77 -0.67 19.59 83 39 100088 SER A, 100040 ARG A 0.26 39.4 -2.45 -0.61 27.69 83 40 100040 ARG A -0.03 45.91 -4.5 -0.42 52 83 41 100040 ARG A, 100043 HIS A 0.9 47.06 -3.85 -0.08 51.8 87 42 100040 ARG A, 100046 GLU A 1.48 50.65 -4 -0.78 50.95 80 43 100040 ARG A, 100046 GLU A, 300018 ARG B 3.93 52.41 -4.17 -0.66 51.3 81 44 100040 ARG A, 100046 GLU A, 300018 ARG B, 100064 ILE A 3.04 56.47 -2 -0.04 38.51 86 45 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A 2.73 59.5 -0.98 -0.14 26.35 87 46 100046 GLU A, 100064 ILE A, 100063 LYS A 2.84 60.4 0.2 -0.04 17.8 90 47 100046 GLU A, 100064 ILE A, 100063 LYS A 3.02 64.82 -0.97 0.09 33.18 84 48 100046 GLU A, 100064 ILE A, 100063 LYS A, 300020 SER B 5.06 65.31 -0.93 -0.18 25.3 92 49 100046 GLU A, 100063 LYS A, 300020 SER B, 100003 LEU B 5.34 65.71 -1.1 -0.35 25.3 80 50 100046 GLU A, 100064 ILE A, 100063 LYS A, 100003 LEU B 5.5 70.29 0.23 0.35 24.92 76 51 100046 GLU A, 100063 LYS A, 100003 LEU B, 100061 ASN A, 100098 PHE B 5.32 71.06 -1.5 -0.4 21.26 76 52 100046 GLU A, 100063 LYS A, 100061 ASN A, 100098 PHE B, 100097 THR B 5.11 71.38 -2.4 -0.78 21.56 90 53 100046 GLU A, 100061 ASN A, 100098 PHE B, 100097 THR B 4.6 71.57 -2.03 -0.87 14.58 96 54 100046 GLU A, 100063 LYS A, 100061 ASN A, 100098 PHE B, 100097 THR B 4.35 71.82 -2.4 -0.78 21.56 90 55 100063 LYS A, 100003 LEU B, 100061 ASN A, 100098 PHE B, 100097 THR B 3.81 72.9 -0.94 -0.32 11.61 84 56 100063 LYS A, 100003 LEU B, 100061 ASN A, 100097 THR B 3.71 73.4 -1.08 -0.2 13.67 84 57 100063 LYS A, 100003 LEU B, 100097 THR B, 100001 ASP B 3.59 75.08 -1.08 -0.27 25.25 79 58 100063 LYS A, 100003 LEU B, 100001 ASP B 3.59 77.07 -1.2 -0.1 33.11 70 59 100063 LYS A, 100003 LEU B, 100001 ASP B, 300072 THR B 3.71 78.05 -1.08 -0.27 25.25 79 60 100003 LEU B, 100001 ASP B, 300072 THR B, 300070 ASP B 3.89 79 -0.98 -0.43 25.3 83 61 100003 LEU B, 300072 THR B, 300070 ASP B, 100001 ASP B 3.84 79.18 -0.2 -0.37 13.72 82 62 100003 LEU B, 300070 ASP B, 100001 ASP B 2.99 81.47 -0.03 -0.23 17.74 70 63 100003 LEU B, 300070 ASP B 2.23 84.45 0.15 0.05 24.92 70 64 100003 LEU B, 300070 ASP B, 100026 SER B 2.11 86.68 -0.17 -0.29 17.17 85 65 100003 LEU B, 300070 ASP B, 100026 SER B, 300024 ARG B 2.36 88.68 -1.25 -0.32 25.88 85 66 300070 ASP B, 100026 SER B, 300024 ARG B 3.23 90.32 -2.93 -0.81 34.46 95 67 300070 ASP B, 300024 ARG B, 100026 SER B 3.6 91.2 -2.8 -0.75 35.03 84 68 300070 ASP B, 300024 ARG B, 100026 SER B, 300069 THR B 3.72 92.83 -2.28 -0.76 26.69 92 69 300024 ARG B, 100026 SER B, 300069 THR B 4.22 94.53 -1.87 -0.66 19.01 95 70 300024 ARG B, 100026 SER B, 300069 THR B, 300026 SER B 4.83 95.66 -1.5 -0.7 15.11 95 71 100026 SER B, 300069 THR B, 300026 SER B 5.33 98.48 -0.5 -0.79 2.81 107 72 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B 5.26 99.52 -0.48 -0.79 2.95 107 73 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B, 300028 SER B 4.87 101.19 -0.46 -0.79 3.04 107 74 100026 SER B, 300027 GLN B, 100027 GLN B, 300028 SER B 4.69 101.35 -1.28 -0.92 2.99 100 75 100026 SER B, 300069 THR B, 100027 GLN B, 300028 SER B 4.08 104.78 -1.35 -0.91 2.56 102 76 300027 GLN B, 100027 GLN B, 300028 SER B 5.32 105.53 -1.57 -0.96 2.86 100 77 100027 GLN B, 300028 SER B, 100028 SER B, 300027 GLN B 5.62 106.61 -2.05 -0.99 3.03 95 78 27 GLN B, 28 SER B, 200027 GLN B, 200028 SER B 6.12 107.37 -2.05 -0.99 3.03 95 79 200027 GLN B, 200028 SER B, 100028 SER B 6.21 107.91 -1.7 -1.01 2.29 106 80 100027 GLN B, 300028 SER B, 100028 SER B 5.02 111.22 -1.7 -1.01 2.29 106 81 100027 GLN B, 300028 SER B, 100028 SER B, 100093 ARG B 4.72 111.39 -2.4 -0.87 14.72 100 82 100027 GLN B, 100028 SER B, 100093 ARG B 3.86 111.82 -2.93 -0.83 19.07 94 83 100027 GLN B, 300028 SER B, 100028 SER B, 100093 ARG B 3.41 112.92 -2.4 -0.87 14.72 100 84 100027 GLN B, 300028 SER B, 100093 ARG B 3.06 118.11 -2.93 -0.83 19.07 94 85 300028 SER B, 100093 ARG B 3.44 118.91 -2.65 -0.7 26.84 100 86 300028 SER B, 100093 ARG B, 100082 TYR C 3.57 120.06 -2.2 -0.09 18.43 83 87 300028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B 3.8 120.48 -2.78 -0.18 26.82 83 88 300028 SER B, 100082 TYR C, 300093 ARG B, 300058 GLN C 3.87 121.54 -2.53 -0.35 14.7 83 89 300028 SER B, 300093 ARG B, 300058 GLN C 3.85 121.77 -2.93 -0.83 19.07 94 90 300028 SER B, 100082 TYR C, 300093 ARG B, 300058 GLN C 3.8 122.49 -2.53 -0.35 14.7 83 91 100082 TYR C, 300093 ARG B, 300058 GLN C 3.8 123.54 -3.1 -0.14 19.05 72 92 100082 TYR C, 300093 ARG B, 300058 GLN C, 300079 GLY C 3.78 125.11 -2.43 -0.3 15.13 72 93 300093 ARG B, 300058 GLN C, 300079 GLY C, 300080 ASP C 3.78 125.48 -2.2 -0.78 15.57 83 94 300093 ARG B, 300058 GLN C, 300079 GLY C, 300082 TYR C 3.8 129.17 -2.43 -0.3 15.13 72 95 100082 TYR C, 300093 ARG B, 300079 GLY C, 300082 TYR C 4.73 130.23 -1.88 0.25 14.65 61 96 100082 TYR C, 300093 ARG B, 300079 GLY C, 300082 TYR C, 79 GLY C 5.1 131.05 -1.58 0.04 12.4 61 97 300093 ARG B, 300079 GLY C, 300082 TYR C, 79 GLY C, 93 ARG B 5.48 131.49 -2.22 -0.27 22.47 72 98 93 ARG B, 200093 ARG B, 82 TYR C, 200079 GLY C, 200082 TYR C 5.87 131.64 -2.4 0.12 22.12 66 99 100093 ARG B, 200093 ARG B, 82 TYR C, 200079 GLY C, 200082 TYR C 6.22 131.97 -2.4 0.12 22.12 66 100 200093 ARG B, 82 TYR C, 200079 GLY C, 200082 TYR C, 100079 GLY C 6.73 132.29 -1.58 0.04 12.4 61 101 300093 ARG B, 300079 GLY C, 300082 TYR C, 79 GLY C, 93 ARG B 6.65 133.57 -2.22 -0.27 22.47 72 102 300093 ARG B, 300082 TYR C, 79 GLY C, 93 ARG B, 28 SER B 6.4 134.07 -2.3 -0.3 22.13 83 103 300093 ARG B, 300082 TYR C, 93 ARG B, 28 SER B 4.14 137.1 -2.78 -0.18 26.82 83 104 300093 ARG B, 300082 TYR C, 28 SER B 3.61 138.63 -2.2 -0.09 18.43 83 105 300093 ARG B, 28 SER B 3.43 139.48 -2.65 -0.7 26.84 100 106 300027 GLN B, 300093 ARG B, 28 SER B 3.17 143.97 -2.93 -0.83 19.07 94 107 300028 SER B, 300027 GLN B, 300093 ARG B, 28 SER B 3.43 144.57 -2.3 -0.82 15.15 94 108 300028 SER B, 300027 GLN B, 300093 ARG B, 28 SER B 3.59 144.89 -2.4 -0.87 14.72 100 109 300028 SER B, 300027 GLN B, 300093 ARG B 3.77 145.28 -2.93 -0.83 19.07 94 110 300028 SER B, 300027 GLN B, 300093 ARG B, 28 SER B 4.47 145.92 -2.4 -0.87 14.72 100 111 300028 SER B, 300027 GLN B, 28 SER B 5.02 149.37 -1.7 -1.01 2.29 106 112 100027 GLN B, 200027 GLN B, 100028 SER B, 200028 SER B 6.16 150.62 -2.05 -0.99 3.03 95 113 300028 SER B, 300027 GLN B, 27 GLN B, 28 SER B 5.94 151.3 -2.05 -0.99 3.03 95 114 300027 GLN B, 27 GLN B, 28 SER B 5.67 152.06 -2.6 -1.06 2.91 95 115 300026 SER B, 300027 GLN B, 28 SER B 5.36 152.81 -1.57 -0.96 2.86 100 116 300026 SER B, 300027 GLN B, 28 SER B, 69 THR B 4.07 155.34 -1.35 -0.91 2.56 102 117 300026 SER B, 300027 GLN B, 28 SER B, 27 GLN B 4.45 155.82 -1.28 -0.92 2.99 100 118 300026 SER B, 28 SER B, 69 THR B, 27 GLN B, 26 SER B 4.79 158.44 -0.46 -0.79 3.04 107 119 300026 SER B, 69 THR B, 27 GLN B, 26 SER B 5.19 159.51 -0.48 -0.79 2.95 107 120 300026 SER B, 69 THR B, 26 SER B 5.27 161.53 -0.5 -0.79 2.81 107 121 300026 SER B, 69 THR B, 26 SER B, 24 ARG B 4.85 162.69 -1.5 -0.7 15.11 95 122 300026 SER B, 69 THR B, 24 ARG B 4.45 163.98 -1.87 -0.66 19.01 95 123 300026 SER B, 69 THR B, 24 ARG B, 70 ASP B 3.87 165.52 -2.28 -0.76 26.69 92 124 300026 SER B, 24 ARG B, 70 ASP B 3.59 166.38 -2.8 -0.75 35.03 84 125 24 ARG B, 70 ASP B, 300026 SER B 3.19 168.58 -2.93 -0.81 34.46 95 126 24 ARG B, 70 ASP B, 300026 SER B, 300003 LEU B 2.52 170.11 -1.25 -0.32 25.88 85 127 70 ASP B, 300026 SER B, 300003 LEU B 2.1 172.93 -0.17 -0.29 17.17 85 128 70 ASP B, 300003 LEU B 2.25 175.81 0.15 0.05 24.92 70 129 70 ASP B, 300003 LEU B, 300001 ASP B 3.05 177.69 -0.03 -0.23 17.74 70 130 70 ASP B, 300003 LEU B, 300001 ASP B, 72 THR B 3.85 177.82 -0.2 -0.37 13.72 82 131 70 ASP B, 300003 LEU B, 72 THR B, 300001 ASP B 3.9 178.65 -0.98 -0.43 25.3 83 132 70 ASP B, 300003 LEU B, 72 THR B, 300001 ASP B, 300063 LYS A 3.88 178.9 -1.56 -0.42 30.14 81 133 300003 LEU B, 72 THR B, 300001 ASP B, 300063 LYS A 3.69 180.35 -1.08 -0.27 25.25 79 134 300003 LEU B, 300001 ASP B, 300063 LYS A 3.58 181.74 -1.2 -0.1 33.11 70 135 300003 LEU B, 300001 ASP B, 300063 LYS A, 300097 THR B 3.54 183.86 -1.08 -0.27 25.25 79 136 300003 LEU B, 300063 LYS A, 300097 THR B, 300061 ASN A 3.66 184.29 -1.08 -0.2 13.67 84 137 300003 LEU B, 300063 LYS A, 300097 THR B, 300061 ASN A, 300098 PHE B 3.77 185.12 -0.94 -0.32 11.61 84 138 300063 LYS A, 300097 THR B, 300061 ASN A, 300098 PHE B, 300046 GLU A 4.34 185.26 -2.4 -0.78 21.56 90 139 300097 THR B, 300061 ASN A, 300098 PHE B, 300046 GLU A 4.61 185.77 -2.03 -0.87 14.58 96 140 300003 LEU B, 300063 LYS A, 300061 ASN A, 300098 PHE B, 300046 GLU A 5.16 186.65 -1.5 -0.4 21.26 76 141 300003 LEU B, 300063 LYS A, 300098 PHE B, 300046 GLU A 5.41 187.88 -1 -0.3 25.73 67 142 300003 LEU B, 300063 LYS A, 300046 GLU A, 300064 ILE A 5.6 191.11 0.23 0.35 24.92 76 143 300003 LEU B, 300063 LYS A, 300046 GLU A, 20 SER B 5.49 192.03 -1.1 -0.35 25.3 80 144 300063 LYS A, 300046 GLU A, 300064 ILE A, 20 SER B 5.21 192.89 -0.93 -0.18 25.3 92 145 300063 LYS A, 300046 GLU A, 300064 ILE A 3.09 197.4 -0.97 0.09 33.18 84 146 300046 GLU A, 300064 ILE A, 300063 LYS A, 18 ARG B 2.73 201.01 -0.98 -0.14 26.35 87 147 300046 GLU A, 300064 ILE A, 18 ARG B, 300040 ARG A 3 204.38 -2 -0.04 38.51 86 148 300046 GLU A, 18 ARG B, 300040 ARG A 3.62 206.49 -4.17 -0.66 51.3 81 149 300046 GLU A, 300064 ILE A, 300040 ARG A 2.27 207.7 -1.17 0.08 34.01 87 150 300046 GLU A, 300040 ARG A 0.97 210.56 -4 -0.78 50.95 80 151 300040 ARG A -0.04 217.24 -4.5 -0.42 52 83 152 300040 ARG A, 300088 SER A 0.26 217.89 -2.45 -0.61 27.69 83 153 300040 ARG A, 300088 SER A, 300091 SER A 0.64 218.2 -1.77 -0.67 19.59 83 154 300040 ARG A, 300088 SER A, 300041 PRO A, 300091 SER A 1.12 218.48 -1.83 -0.57 14.66 86 155 300040 ARG A, 300088 SER A, 300091 SER A, 300041 PRO A 1.66 219.33 -1.73 -0.53 15.09 70 156 300040 ARG A, 300088 SER A, 300041 PRO A 2.81 221.46 -2.17 -0.44 18.99 70 157 300040 ARG A, 300041 PRO A, 300088 SER A 4.17 223.65 -2.3 -0.49 18.42 86 158 300041 PRO A, 300088 SER A 4.95 225.16 -1.2 -0.53 1.63 87 159 300041 PRO A, 300091 SER A, 300088 SER A 4.83 225.69 -1.07 -0.68 1.64 97 160 300041 PRO A, 300091 SER A, 300088 SER A, 300175 LEU A 4.69 226.15 0.15 -0.22 1.26 86 161 300041 PRO A, 300088 SER A, 300175 LEU A, 300180 TYR A 4.39 226.74 0.02 0.3 1.25 69 162 300091 SER A, 300088 SER A, 300175 LEU A, 300180 TYR A 4.25 226.93 0.23 0.08 1.27 84 163 300091 SER A, 300088 SER A, 300180 TYR A 4.14 227.03 -0.97 -0.28 1.65 94 164 300091 SER A, 300088 SER A 4.07 227.19 -0.8 -0.97 1.67 117 165 300091 SER A, 300088 SER A, 300180 TYR A 4.03 227.65 -0.97 -0.28 1.65 94 166 300041 PRO A, 300088 SER A, 300175 LEU A, 300180 TYR A 4.03 228.45 0.02 0.3 1.25 69 167 300041 PRO A, 300091 SER A, 300175 LEU A, 300180 TYR A 4.07 229.49 0.02 0.3 1.25 69 168 300041 PRO A, 300175 LEU A, 300180 TYR A 4.12 233.05 0.3 0.72 1.11 54 169 300041 PRO A, 300175 LEU A, 300180 TYR A, 300173 ALA A 4.5 233.25 0.13 0.34 1.68 54 170 300041 PRO A, 300175 LEU A, 300180 TYR A 4.32 233.95 0.3 0.72 1.11 54 171 300041 PRO A, 300175 LEU A, 300180 TYR A, 300173 ALA A 3.83 234.74 0.13 0.34 1.68 54 172 300041 PRO A, 300180 TYR A, 300173 ALA A 3.54 236.66 -1.1 0.07 2.19 54 173 300041 PRO A, 300180 TYR A, 300173 ALA A, 300153 GLU A 3.46 238.74 -1.7 -0.23 14.12 61 174 300041 PRO A, 300173 ALA A, 300153 GLU A, 300172 PRO A 3.33 241.16 -1.78 -0.53 14.11 64 175 300041 PRO A, 300153 GLU A, 300172 PRO A, 300041 ASN B 3.13 241.77 -2.55 -0.52 14.11 74 176 300041 PRO A, 300153 GLU A, 300172 PRO A, 300041 ASN B 3.02 242.12 -2.55 -0.52 14.11 74 177 300153 GLU A, 300172 PRO A, 300041 ASN B 2.79 243.04 -2.87 -0.67 18.29 79 178 300173 ALA A, 300153 GLU A, 300172 PRO A, 300041 ASN B 2.51 246.35 -2.25 -0.7 14.56 79 179 300173 ALA A, 300153 GLU A, 300041 ASN B, 300172 PRO A 2.51 246.86 -1.95 -0.88 15.01 90 180 300173 ALA A, 300153 GLU A, 300041 ASN B, 300172 PRO A, 300182 LEU A 2.53 247.18 -0.8 -0.47 12.03 78 181 300153 GLU A, 300041 ASN B, 300172 PRO A, 300182 LEU A, 300171 PHE A 2.56 247.4 -0.8 -0.47 12.03 78 182 300041 ASN B, 300172 PRO A, 300182 LEU A, 300171 PHE A 2.62 248.33 -0.13 -0.31 2.57 79 183 300041 ASN B, 300182 LEU A, 300171 PHE A 2.76 248.54 -0.03 -0.14 2.3 79 184 300041 ASN B, 300182 LEU A, 300170 THR A 2.76 248.71 -0.13 -0.13 1.72 88 185 300041 ASN B, 300182 LEU A, 300170 THR A, 300155 VAL A 2.72 249.9 -0.2 -0.3 2.14 88 186 300041 ASN B, 300170 THR A, 300155 VAL A 2.77 253.92 -1.53 -0.78 2.81 105 187 300041 ASN B, 300170 THR A, 300156 THR A, 300157 VAL A 3.04 254.15 -1.33 -0.78 2.52 106 188 300041 ASN B, 300156 THR A, 300157 VAL A 3.1 254.29 -1.53 -0.78 2.81 105 189 300041 ASN B, 300170 THR A, 300156 THR A, 300157 VAL A 3.2 254.62 -1.33 -0.78 2.52 106 190 300041 ASN B, 300170 THR A, 300155 VAL A 3.26 254.8 -1.53 -0.78 2.81 105 191 300041 ASN B, 300170 THR A, 300157 VAL A 3.28 255.03 -1.53 -0.78 2.81 105 192 300041 ASN B, 300170 THR A, 300156 THR A, 300157 VAL A 3.24 255.56 -1.33 -0.78 2.52 106 193 300041 ASN B, 300170 THR A, 300157 VAL A 3.4 258.4 -1.53 -0.78 2.81 105 194 300041 ASN B, 300170 THR A, 300157 VAL A, 300040 THR B 3.95 259.49 -1.25 -0.79 2.95 105 195 300170 THR A, 300157 VAL A, 300040 THR B, 300168 VAL A 4.26 259.71 0.68 -0.31 2.14 102 196 300170 THR A, 300157 VAL A, 300040 THR B, 300168 VAL A, 300169 LYS B 4.51 259.85 -1.16 -0.72 12.26 89 197 300170 THR A, 300157 VAL A, 300168 VAL A, 300169 LYS B 4 260.54 -0.2 -0.21 13.67 92 198 300157 VAL A, 300168 VAL A, 300169 LYS B, 300165 SER A 3.24 261.42 -0.13 -0.22 14.1 85 199 300168 VAL A, 300169 LYS B, 300165 SER A 1.75 265.6 -0.03 -0.03 17.67 85 200 300168 VAL A, 300169 LYS B, 300165 SER A 1.24 267.31 -1.57 -0.67 18.75 72 201 300168 VAL A, 300169 LYS B, 300165 SER A, 300167 GLY A 1.26 268.77 -1.28 -0.7 14.91 72 202 300168 VAL A, 300169 LYS B, 300167 GLY A 1.72 269.61 -1.57 -0.67 18.75 72 203 300168 VAL A, 300169 LYS B, 300167 GLY A, 300169 HIS A 1.87 269.79 -1.98 -0.44 26.97 81 204 300168 VAL A, 300169 LYS B, 300167 GLY A, 300169 HIS A 1.92 269.97 -1.98 -0.44 26.97 81 205 300168 VAL A, 300169 LYS B, 300167 GLY A, 300169 HIS A 1.98 270.08 -1.98 -0.44 26.97 81 206 300168 VAL A, 300169 LYS B, 300167 GLY A, 300169 HIS A 1.87 270.55 -1.98 -0.44 26.97 81 207 300169 LYS B, 300167 GLY A, 300169 HIS A, 300167 ASP B 1.77 271.3 -2.75 -0.5 38.55 83 208 300169 LYS B, 300167 GLY A, 300167 ASP B 1.69 272.75 -2.6 -0.75 34.19 79 209 300169 LYS B, 300167 GLY A 1.68 273.51 -2.15 -0.61 26.44 72 210 300169 LYS B, 300167 GLY A, 300166 SER A 1.69 274.54 -1.57 -0.67 18.75 72 211 300169 LYS B, 300167 GLY A, 300166 SER A 1.79 278.2 -1.7 -0.73 18.18 94 212 300169 LYS B, 300167 GLY A, 300166 SER A, 300170 ASP B 3 279.53 -2.15 -0.81 26.06 91 213 300167 GLY A, 300166 SER A, 300170 ASP B, 300169 LYS B 4.21 280.01 -1.28 -0.9 14.53 101 214 300167 GLY A, 300166 SER A, 300170 ASP B 4.4 280.92 -1.57 -0.94 18.25 101 215 300167 GLY A, 300166 SER A, 300170 ASP B, 300140 MET A 4.75 281.97 -0.7 -0.45 14.05 98 216 300167 GLY A, 300170 ASP B, 300140 MET A 4.13 283.62 -0.67 -0.28 18.17 89 217 300167 GLY A, 300170 ASP B, 300140 MET A, 300138 ASN B 3.96 284.91 -1.38 -0.4 14.47 94 218 300170 ASP B, 300140 MET A, 300138 ASN B 4 286.32 -1.7 -0.27 18.17 94 219 300140 MET A, 300138 ASN B, 300187 THR A 3.58 288.37 -0.77 -0.18 2.16 101 220 300140 MET A, 300138 ASN B, 300187 THR A, 300114 THR B 3.14 289.92 -0.75 -0.33 2.03 102 221 300140 MET A, 300187 THR A, 300114 THR B 2.94 291.86 0.17 -0.18 1.58 102 222 300140 MET A, 300114 THR B 2.81 293.28 0.6 0.12 1.55 100 223 300140 MET A, 300114 THR B, 300138 ASN A 2.76 293.63 -0.77 -0.18 2.16 101 224 300140 MET A, 300114 THR B, 300138 ASN A, 300139 SER A 2.61 294.67 -0.78 -0.38 2.04 105 225 300140 MET A, 300114 THR B, 300138 ASN A 2.56 297.72 -0.77 -0.18 2.16 101 226 300114 THR B, 300138 ASN A 2.6 300.92 -2.1 -0.77 2.52 105 227 300114 THR B, 300138 ASN A, 300113 PRO B, 300207 LYS B 2.74 301.44 -2.13 -0.69 14.48 94 228 300114 THR B, 300138 ASN A, 300207 LYS B 2.79 303.3 -2.7 -0.65 18.18 94 229 300114 THR B, 300138 ASN A, 300207 LYS B, 300115 VAL B 2.87 304.37 -2.13 -0.69 14.48 94 230 300114 THR B, 300138 ASN A, 300207 LYS B, 300136 GLN A 2.89 305.25 -2.13 -0.69 14.48 94 231 300114 THR B, 300138 ASN A, 300207 LYS B, 300115 VAL B, 300136 GLN A 2.8 306.25 -1.78 -0.71 12.26 94 232 300114 THR B, 300207 LYS B, 300115 VAL B, 300136 GLN A 2.63 306.71 -1.35 -0.7 14.48 89 233 300114 THR B, 300138 ASN A, 300207 LYS B, 300115 VAL B, 300136 GLN A 2.57 307.03 -1.78 -0.71 12.26 94 234 300114 THR B, 300207 LYS B, 300115 VAL B, 300136 GLN A, 300116 SER B 2.52 307.38 -1.24 -0.75 11.92 98 235 300114 THR B, 300115 VAL B, 300136 GLN A, 300116 SER B, 300140 MET A 2.4 308.93 -0.54 -0.83 2.69 112 236 300207 LYS B, 300115 VAL B, 300136 GLN A, 300116 SER B 2.37 309.61 -1.38 -0.75 14.48 94 237 300115 VAL B, 300136 GLN A, 300116 SER B 2.33 310.8 -0.53 -0.86 2.81 117 238 300115 VAL B, 300136 GLN A, 300116 SER B, 300135 ALA A 2.3 311.5 0.05 -0.64 2.11 108 239 300115 VAL B, 300116 SER B, 300135 ALA A 2.28 312.68 0.2 -0.58 1.68 108 240 300116 SER B, 300135 ALA A, 300117 ILE B 1.88 315.05 0.2 -0.58 1.68 108 241 300135 ALA A, 300117 ILE B, 300116 SER B 1.8 315.55 0.33 -0.53 2.25 100 242 300135 ALA A, 300116 SER B, 300117 ILE B 1.67 316.54 1.97 0.34 1.17 101 243 300135 ALA A, 300117 ILE B 1.61 317.68 3.15 0.92 0.07 101 244 300135 ALA A, 300117 ILE B, 300208 SER B 1.61 318.25 1.97 0.34 1.17 101 245 300135 ALA A, 300117 ILE B, 300208 SER B, 300132 GLY A 1.63 319.15 1.38 0.06 1.72 101 246 300135 ALA A, 300117 ILE B, 300132 GLY A 1.7 319.64 1.97 0.34 1.17 101 247 300117 ILE B, 300132 GLY A, 300209 PHE B 1.67 320.95 2.3 0.79 1.29 77 248 300117 ILE B, 300132 GLY A, 300209 PHE B, 300119 PRO B 1.48 321.42 0.1 -0.09 2.17 54 249 300117 ILE B, 300132 GLY A, 300119 PRO B 1.44 321.57 -0.8 -0.56 2.78 58 250 300117 ILE B, 300132 GLY A, 300119 PRO B 1.42 321.79 0.83 0.31 1.7 80 251 300117 ILE B, 300132 GLY A, 300119 PRO B 1.46 322.26 -0.8 -0.56 2.78 58 252 300132 GLY A, 300209 PHE B, 300119 PRO B 1.52 326.46 0.27 0.15 1.77 54 253 300132 GLY A, 300209 PHE B, 300119 PRO B, 300133 SER A, 300210 ASN B 2.01 327.17 0 -0.23 2.41 54 254 300132 GLY A, 300119 PRO B, 300133 SER A, 300210 ASN B, 300218 ARG A 2.25 328.32 -1.46 -0.58 12.74 70 255 300119 PRO B, 300210 ASN B, 300218 ARG A 2.02 329.88 -2.17 -0.44 18.99 70 256 300209 PHE B, 300119 PRO B, 300210 ASN B, 300218 ARG A 2 330.39 -0.93 0.01 14.33 64 257 300119 PRO B, 300210 ASN B, 300218 ARG A, 300211 ARG B 1.99 331 -1.73 -0.53 15.09 70 258 300119 PRO B, 300210 ASN B, 300218 ARG A, 300211 ARG B, 300186 TYR B 2.07 331.36 -1.64 -0.2 12.39 63 259 300119 PRO B, 300218 ARG A, 300211 ARG B, 300186 TYR B 2.15 333.06 -1.95 -0.05 14.64 63 260 300218 ARG A, 300186 TYR B, 300211 ARG B 2.3 334.64 -3.43 0.09 35.2 72 261 300218 ARG A, 300186 TYR B, 300211 ARG B, 300125 LEU B 2.41 334.64 -1.63 0.35 26.44 67 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
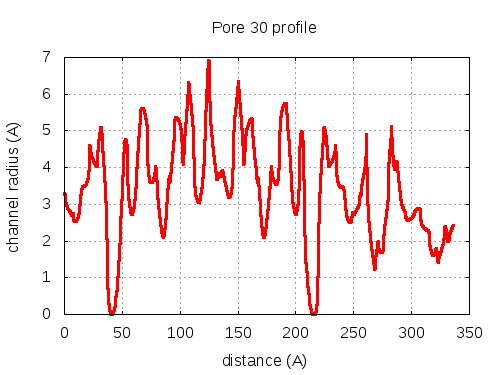
Unique lining residues set - all
100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B, 100155 VAL A, 100182 LEU A, 100171 PHE A, 100172 PRO A, 100153 GLU A, 100173 ALA A, 100172 PRO A, 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A, 100091 SER A, 100088 SER A, 100040 ARG A, 100091 SER A, 100043 HIS A, 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A, 100063 LYS A, 300020 SER B, 100003 LEU B, 100061 ASN A, 100098 PHE B, 100097 THR B, 100001 ASP B, 300072 THR B, 300070 ASP B, 100001 ASP B, 100026 SER B, 300024 ARG B, 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B, 300028 SER B, 100027 GLN B, 300028 SER B, 100028 SER B, 300027 GLN B, 27 GLN B, 28 SER B, 200027 GLN B, 200028 SER B, 100028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 300079 GLY C, 100079 GLY C, 93 ARG B, 79 GLY C, 82 TYR C, 200079 GLY C, 300082 TYR C, 200093 ARG B, 200082 TYR C, 100058 GLN C, 100080 ASP C, 28 SER B, 200058 GLN C, 200080 ASP C, 26 SER B, 200069 THR B, 200027 GLN B, 200026 SER B, 200028 SER B, 200024 ARG B, 200070 ASP B, 26 SER B, 3 LEU B, 1 ASP B, 200072 THR B, 1 ASP B, 63 LYS A, 97 THR B, 2 ILE B, 98 PHE B, 61 ASN A, 46 GLU A, 64 ILE A, 200020 SER B, 63 LYS A, 200018 ARG B, 40 ARG A, 88 SER A, 91 SER A, 41 PRO A, 91 SER A, 88 SER A, 175 LEU A, 180 TYR A, 173 ALA A, 153 GLU A, 172 PRO A, 41 ASN B, 172 PRO A, 182 LEU A, 171 PHE A, 170 THR A, 155 VAL A, 156 THR A, 157 VAL A, 40 THR B, 168 VAL A, 168 VAL A, 169 LYS B, 165 SER A, 167 GLY A, 169 HIS A, 167 ASP B, 166 SER A, 166 SER A, 170 ASP B, 169 LYS B, 140 MET A, 138 ASN B, 187 THR A, 114 THR B, 138 ASN A, 139 SER A, 207 LYS B, 115 VAL B, 136 GLN A, 116 SER B, 140 MET A, 135 ALA A, 117 ILE B, 116 SER B, 117 ILE B, 208 SER B, 132 GLY A, 209 PHE B, 119 PRO B, 210 ASN B, 133 SER A, 218 ARG A, 211 ARG B, 186 TYR B, 211 ARG B, 125 LEU B
Unique lining residues set - sidechains
100156 THR A, 100170 THR A, 100041 ASN B, 100182 LEU A, 100153 GLU A, 100172 PRO A, 100041 PRO A, 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A, 100040 ARG A, 100043 HIS A, 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A, 300020 SER B, 100003 LEU B, 100061 ASN A, 100097 THR B, 100001 ASP B, 300072 THR B, 300070 ASP B, 100026 SER B, 300024 ARG B, 300069 THR B, 100027 GLN B, 300028 SER B, 300027 GLN B, 27 GLN B, 200027 GLN B, 200028 SER B, 100028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 93 ARG B, 82 TYR C, 300082 TYR C, 200093 ARG B, 200082 TYR C, 100058 GLN C, 28 SER B, 200058 GLN C, 200069 THR B, 200024 ARG B, 200070 ASP B, 26 SER B, 3 LEU B, 200072 THR B, 1 ASP B, 63 LYS A, 97 THR B, 61 ASN A, 46 GLU A, 64 ILE A, 200020 SER B, 200018 ARG B, 40 ARG A, 41 PRO A, 91 SER A, 88 SER A, 175 LEU A, 180 TYR A, 153 GLU A, 172 PRO A, 41 ASN B, 182 LEU A, 170 THR A, 156 THR A, 168 VAL A, 169 LYS B, 169 HIS A, 167 ASP B, 166 SER A, 170 ASP B, 140 MET A, 138 ASN B, 187 THR A, 114 THR B, 138 ASN A, 139 SER A, 207 LYS B, 116 SER B, 135 ALA A, 117 ILE B, 209 PHE B, 119 PRO B, 218 ARG A, 186 TYR B, 211 ARG B, 125 LEU B
Physicochemical properties of lining side-chains
Charge: 6 (16-10)
Hydropathy: -1.3
Hydrophobicity: -0.41
Polarity: 13.87
Mutability: 87
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B 3.27 0.28 -1.33 -0.78 2.52 106 2 100157 VAL A, 100170 THR A, 100041 ASN B 3.27 0.43 -1.53 -0.78 2.81 105 3 100170 THR A, 100041 ASN B, 100155 VAL A 3.25 0.64 -1.53 -0.78 2.81 105 4 100157 VAL A, 100170 THR A, 100041 ASN B 3.23 0.82 -1.53 -0.78 2.81 105 5 100156 THR A, 100157 VAL A, 100170 THR A, 100041 ASN B 3.2 0.99 -1.33 -0.78 2.52 106 6 100156 THR A, 100157 VAL A, 100041 ASN B 3.05 1.56 -1.53 -0.78 2.81 105 7 100157 VAL A, 100170 THR A, 100041 ASN B, 100155 VAL A 2.98 2.26 -1.25 -0.79 2.95 105 8 100170 THR A, 100041 ASN B, 100155 VAL A 2.71 5.67 -1.53 -0.78 2.81 105 9 100170 THR A, 100041 ASN B, 100155 VAL A, 100182 LEU A 2.63 6.65 -0.2 -0.3 2.14 88 10 100170 THR A, 100041 ASN B, 100182 LEU A 2.71 7.07 -0.13 -0.13 1.72 88 11 100041 ASN B, 100182 LEU A, 100171 PHE A 2.74 7.26 -0.03 -0.14 2.3 79 12 100041 ASN B, 100182 LEU A, 100171 PHE A, 100172 PRO A 2.6 8.03 -0.13 -0.31 2.57 79 13 100041 ASN B, 100182 LEU A, 100171 PHE A, 100172 PRO A, 100153 GLU A 2.6 8.32 -0.8 -0.47 12.03 78 14 100041 ASN B, 100182 LEU A, 100172 PRO A, 100153 GLU A, 100173 ALA A 2.61 8.8 -0.8 -0.47 12.03 78 15 100041 ASN B, 100172 PRO A, 100153 GLU A, 100173 ALA A 2.63 10.14 -1.95 -0.88 15.01 90 16 100041 ASN B, 100153 GLU A, 100173 ALA A, 100172 PRO A 2.7 12.67 -2.25 -0.7 14.56 79 17 100041 ASN B, 100153 GLU A, 100172 PRO A 2.91 13.46 -2.87 -0.67 18.29 79 18 100041 ASN B, 100153 GLU A, 100172 PRO A, 100041 PRO A 3.04 13.76 -2.55 -0.52 14.11 74 19 100041 ASN B, 100153 GLU A, 100172 PRO A, 100041 PRO A 3.12 14.44 -2.55 -0.52 14.11 74 20 100153 GLU A, 100172 PRO A, 100041 PRO A 3.27 14.82 -2.23 -0.44 17.69 64 21 100153 GLU A, 100173 ALA A, 100172 PRO A, 100041 PRO A 3.34 17.02 -1.78 -0.53 14.11 64 22 100153 GLU A, 100173 ALA A, 100041 PRO A, 100180 TYR A 3.72 18.63 -1.7 -0.23 14.12 61 23 100173 ALA A, 100041 PRO A, 100180 TYR A 3.93 20.99 -1.1 0.07 2.19 54 24 100173 ALA A, 100041 PRO A, 100180 TYR A, 100175 LEU A 4.16 21.44 0.13 0.34 1.68 54 25 100041 PRO A, 100180 TYR A, 100175 LEU A 4.32 22.04 0.3 0.72 1.11 54 26 100173 ALA A, 100041 PRO A, 100180 TYR A, 100175 LEU A 4.32 22.47 0.13 0.34 1.68 54 27 100041 PRO A, 100180 TYR A, 100175 LEU A 4.19 27.1 0.3 0.72 1.11 54 28 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A, 100091 SER A 4.17 27.8 -0.06 0.08 1.67 69 29 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A 4.18 28.14 0.23 0.08 1.27 84 30 100091 SER A, 100088 SER A 4.2 28.24 -0.8 -0.97 1.67 117 31 100180 TYR A, 100091 SER A, 100088 SER A 4.25 28.6 -0.97 -0.28 1.65 94 32 100180 TYR A, 100175 LEU A, 100091 SER A, 100088 SER A 4.44 28.88 0.23 0.08 1.27 84 33 100041 PRO A, 100180 TYR A, 100175 LEU A, 100088 SER A 4.59 29.27 0.02 0.3 1.25 69 34 100041 PRO A, 100175 LEU A, 100091 SER A, 100088 SER A 4.77 29.77 0.15 -0.22 1.26 86 35 100041 PRO A, 100091 SER A, 100088 SER A 4.96 30.88 -1.07 -0.68 1.64 97 36 100041 PRO A, 100088 SER A 5.29 31.77 -1.2 -0.53 1.63 87 37 100041 PRO A, 100088 SER A, 100040 ARG A 4.39 34.23 -2.3 -0.49 18.42 86 38 100041 PRO A, 100088 SER A, 100040 ARG A 2.88 36.66 -2.17 -0.44 18.99 70 39 100041 PRO A, 100088 SER A, 100040 ARG A, 100091 SER A 2.62 37.02 -1.73 -0.53 15.09 70 40 100088 SER A, 100040 ARG A, 100091 SER A 2.37 38.62 -1.77 -0.67 19.59 83 41 100088 SER A, 100040 ARG A 2.43 40.01 -2.45 -0.61 27.69 83 42 100040 ARG A 2.54 45.13 -4.5 -0.42 52 83 43 100040 ARG A, 100043 HIS A 3.01 46.46 -3.85 -0.08 51.8 87 44 100040 ARG A, 100046 GLU A 3.16 50.3 -4 -0.78 50.95 80 45 100040 ARG A, 100046 GLU A, 300018 ARG B 3.52 52.76 -4.17 -0.66 51.3 81 46 100040 ARG A, 100046 GLU A, 300018 ARG B, 100064 ILE A 3.62 56.24 -2 -0.04 38.51 86 47 100046 GLU A, 300018 ARG B, 100064 ILE A 3.61 56.92 -1.17 0.08 34.01 87 48 100046 GLU A, 300018 ARG B, 100064 ILE A, 100063 LYS A 3.55 60.14 -0.98 -0.14 26.35 87 49 100046 GLU A, 100064 ILE A, 100063 LYS A 3.54 64.66 -0.97 0.09 33.18 84 50 100046 GLU A, 100064 ILE A, 100063 LYS A, 300020 SER B 3.84 65.24 -0.93 -0.18 25.3 92 51 100046 GLU A, 100063 LYS A, 300020 SER B, 100003 LEU B 3.93 66.39 -1.1 -0.35 25.3 80 52 100046 GLU A, 100064 ILE A, 100063 LYS A, 100003 LEU B 4.1 70.13 0.23 0.35 24.92 76 53 100046 GLU A, 100063 LYS A, 100003 LEU B, 100061 ASN A, 100098 PHE B 4.21 70.97 -1.5 -0.4 21.26 76 54 100046 GLU A, 100063 LYS A, 100061 ASN A, 100098 PHE B, 100097 THR B 4.19 71.34 -2.4 -0.78 21.56 90 55 100046 GLU A, 100061 ASN A, 100098 PHE B, 100097 THR B 4.05 71.55 -2.03 -0.87 14.58 96 56 100046 GLU A, 100063 LYS A, 100061 ASN A, 100098 PHE B, 100097 THR B 3.95 71.79 -2.4 -0.78 21.56 90 57 100063 LYS A, 100003 LEU B, 100061 ASN A, 100098 PHE B, 100097 THR B 3.67 72.88 -0.94 -0.32 11.61 84 58 100063 LYS A, 100003 LEU B, 100061 ASN A, 100097 THR B 3.61 73.38 -1.08 -0.2 13.67 84 59 100063 LYS A, 100003 LEU B, 100097 THR B, 100001 ASP B 3.58 75.07 -1.08 -0.27 25.25 79 60 100063 LYS A, 100003 LEU B, 100001 ASP B 3.67 77.08 -1.2 -0.1 33.11 70 61 100063 LYS A, 100003 LEU B, 100001 ASP B, 300072 THR B 3.89 78.06 -1.08 -0.27 25.25 79 62 100003 LEU B, 100001 ASP B, 300072 THR B, 300070 ASP B 4.01 79.03 -0.98 -0.43 25.3 83 63 100003 LEU B, 300072 THR B, 300070 ASP B, 100001 ASP B 4 79.22 -0.2 -0.37 13.72 82 64 100003 LEU B, 300070 ASP B, 100001 ASP B 2.9 81.11 -0.03 -0.23 17.74 70 65 100003 LEU B, 300070 ASP B 2.14 84.09 0.15 0.05 24.92 70 66 100003 LEU B, 300070 ASP B, 100026 SER B 2.14 86.92 -0.17 -0.29 17.17 85 67 100003 LEU B, 300070 ASP B, 100026 SER B, 300024 ARG B 2.74 88.92 -1.25 -0.32 25.88 85 68 300070 ASP B, 100026 SER B, 300024 ARG B 3.3 90.31 -2.93 -0.81 34.46 95 69 300070 ASP B, 300024 ARG B, 100026 SER B 3.62 91.45 -2.8 -0.75 35.03 84 70 300070 ASP B, 300024 ARG B, 100026 SER B, 300069 THR B 4.34 93.28 -2.28 -0.76 26.69 92 71 300024 ARG B, 100026 SER B, 300069 THR B 4.62 94.42 -1.87 -0.66 19.01 95 72 300024 ARG B, 100026 SER B, 300069 THR B, 300026 SER B 4.5 95.53 -1.5 -0.7 15.11 95 73 100026 SER B, 300069 THR B, 300026 SER B 4.31 98.26 -0.5 -0.79 2.81 107 74 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B 4.26 99.33 -0.48 -0.79 2.95 107 75 100026 SER B, 300069 THR B, 300026 SER B, 300027 GLN B, 300028 SER B 4.22 101.17 -0.46 -0.79 3.04 107 76 100026 SER B, 300027 GLN B, 100027 GLN B, 300028 SER B 4.25 101.54 -1.28 -0.92 2.99 100 77 100026 SER B, 300069 THR B, 100027 GLN B, 300028 SER B 4.39 104.75 -1.35 -0.91 2.56 102 78 300027 GLN B, 100027 GLN B, 300028 SER B 6 105.52 -1.57 -0.96 2.86 100 79 100027 GLN B, 300028 SER B, 100028 SER B, 300027 GLN B 6.11 106.61 -2.05 -0.99 3.03 95 80 27 GLN B, 28 SER B, 200027 GLN B, 200028 SER B 5.88 107.39 -2.05 -0.99 3.03 95 81 100027 GLN B, 300028 SER B, 200028 SER B, 100028 SER B 5.75 107.95 -1.48 -1 2.14 108 82 100027 GLN B, 300028 SER B, 100028 SER B 5.07 111.25 -1.7 -1.01 2.29 106 83 100027 GLN B, 300028 SER B, 100028 SER B, 100093 ARG B 4.97 111.4 -2.4 -0.87 14.72 100 84 100027 GLN B, 100028 SER B, 100093 ARG B 4.67 111.85 -2.93 -0.83 19.07 94 85 100027 GLN B, 300028 SER B, 100028 SER B, 100093 ARG B 4.55 112.3 -2.4 -0.87 14.72 100 86 100027 GLN B, 300028 SER B, 100028 SER B, 100093 ARG B 4.43 113.01 -2.3 -0.82 15.15 94 87 100027 GLN B, 300028 SER B, 100093 ARG B 3.61 118.46 -2.93 -0.83 19.07 94 88 300028 SER B, 100093 ARG B, 100082 TYR C 3.65 120.36 -2.2 -0.09 18.43 83 89 300028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B 4.25 122.88 -2.78 -0.18 26.82 83 90 300028 SER B, 100093 ARG B, 100082 TYR C, 300093 ARG B, 300079 GLY C 5.99 123.83 -2.3 -0.3 22.13 83 91 100093 ARG B, 100082 TYR C, 300093 ARG B, 300079 GLY C, 100079 GLY C 6.45 124.55 -2.22 -0.27 22.47 72 92 93 ARG B, 79 GLY C, 82 TYR C, 200079 GLY C, 300082 TYR C 6.58 124.81 -1.58 0.04 12.4 61 93 93 ARG B, 79 GLY C, 82 TYR C, 300082 TYR C, 200093 ARG B 6.27 125.36 -2.4 0.12 22.12 66 94 300093 ARG B, 93 ARG B, 79 GLY C, 82 TYR C, 300082 TYR C 6.06 126.05 -2.4 0.12 22.12 66 95 100093 ARG B, 100082 TYR C, 300079 GLY C, 100079 GLY C, 200082 TYR C 5.54 128.13 -1.58 0.04 12.4 61 96 100093 ARG B, 100082 TYR C, 100079 GLY C, 100058 GLN C 4.38 131.33 -2.43 -0.3 15.13 72 97 100093 ARG B, 100079 GLY C, 100058 GLN C 4.17 131.7 -2.8 -0.77 19.64 83 98 100093 ARG B, 100079 GLY C, 100058 GLN C, 100080 ASP C 3.95 132.74 -2.2 -0.78 15.57 83 99 100093 ARG B, 100079 GLY C, 200082 TYR C, 100058 GLN C 3.97 134.53 -2.43 -0.3 15.13 72 100 100093 ARG B, 100082 TYR C, 100079 GLY C, 200082 TYR C, 100058 GLN C 4.38 135.59 -2.2 -0.02 12.43 66 101 100093 ARG B, 100082 TYR C, 100079 GLY C, 200093 ARG B, 200082 TYR C 4.73 137.46 -2.4 0.12 22.12 66 102 100093 ARG B, 100079 GLY C, 200079 GLY C, 200093 ARG B, 200082 TYR C 5.53 138.06 -2.22 -0.27 22.47 72 103 300093 ARG B, 300079 GLY C, 93 ARG B, 79 GLY C, 300082 TYR C 5.92 138.53 -2.22 -0.27 22.47 72 104 300093 ARG B, 300079 GLY C, 93 ARG B, 300082 TYR C, 28 SER B 6.49 138.72 -2.3 -0.3 22.13 83 105 100082 TYR C, 300093 ARG B, 300079 GLY C, 93 ARG B, 300082 TYR C 6.59 139.19 -2.4 0.12 22.12 66 106 100093 ARG B, 100082 TYR C, 300093 ARG B, 300079 GLY C, 300082 TYR C 6.53 139.99 -2.4 0.12 22.12 66 107 100079 GLY C, 82 TYR C, 200079 GLY C, 200093 ARG B, 200082 TYR C 5.97 142.19 -1.58 0.04 12.4 61 108 200079 GLY C, 200093 ARG B, 200082 TYR C, 200058 GLN C 3.66 145.24 -2.43 -0.3 15.13 72 109 200079 GLY C, 200093 ARG B, 200058 GLN C, 200080 ASP C 3.11 146.03 -2.2 -0.78 15.57 83 110 82 TYR C, 200079 GLY C, 200093 ARG B, 200058 GLN C 2.96 147.04 -2.43 -0.3 15.13 72 111 82 TYR C, 200093 ARG B, 200058 GLN C 3.37 148.19 -3.1 -0.14 19.05 72 112 200028 SER B, 82 TYR C, 200093 ARG B, 200058 GLN C 3.88 148.72 -2.53 -0.35 14.7 83 113 200028 SER B, 200093 ARG B, 200058 GLN C 3.86 148.96 -2.93 -0.83 19.07 94 114 200028 SER B, 82 TYR C, 200093 ARG B, 200058 GLN C 3.17 150.38 -2.53 -0.35 14.7 83 115 200028 SER B, 93 ARG B, 82 TYR C, 200093 ARG B 2.96 150.9 -2.78 -0.18 26.82 83 116 200028 SER B, 93 ARG B, 82 TYR C 2.6 152.41 -2.2 -0.09 18.43 83 117 200028 SER B, 93 ARG B 2.49 153.27 -2.65 -0.7 26.84 100 118 27 GLN B, 200028 SER B, 93 ARG B 2.45 157.65 -2.93 -0.83 19.07 94 119 27 GLN B, 28 SER B, 200028 SER B, 93 ARG B 3.68 158.29 -2.3 -0.82 15.15 94 120 27 GLN B, 200028 SER B, 93 ARG B, 28 SER B 4.04 158.62 -2.4 -0.87 14.72 100 121 27 GLN B, 93 ARG B, 28 SER B 4.42 159.03 -2.93 -0.83 19.07 94 122 27 GLN B, 200028 SER B, 93 ARG B, 28 SER B 5.5 159.71 -2.4 -0.87 14.72 100 123 27 GLN B, 200028 SER B, 28 SER B 6.08 163.16 -1.7 -1.01 2.29 106 124 100027 GLN B, 300028 SER B, 100028 SER B, 300027 GLN B 5.66 164.48 -2.05 -0.99 3.03 95 125 27 GLN B, 28 SER B, 200027 GLN B, 200028 SER B 5.38 165.2 -2.05 -0.99 3.03 95 126 27 GLN B, 200027 GLN B, 200028 SER B 5.11 165.97 -2.6 -1.06 2.91 95 127 27 GLN B, 200028 SER B, 26 SER B 4.87 166.7 -1.57 -0.96 2.86 100 128 27 GLN B, 200028 SER B, 26 SER B, 200069 THR B 4.52 169.13 -1.35 -0.91 2.56 102 129 27 GLN B, 200028 SER B, 26 SER B, 200027 GLN B 5.12 169.32 -1.28 -0.92 2.99 100 130 200028 SER B, 26 SER B, 200069 THR B, 200027 GLN B 5.2 169.76 -0.58 -0.84 2.52 112 131 26 SER B, 200069 THR B, 200027 GLN B, 200026 SER B, 200028 SER B 5.25 172.62 -0.46 -0.79 3.04 107 132 26 SER B, 200069 THR B, 200026 SER B 5 175.39 -0.5 -0.79 2.81 107 133 26 SER B, 200069 THR B, 200026 SER B, 200024 ARG B 4.42 176.17 -1.5 -0.7 15.11 95 134 26 SER B, 200069 THR B, 200024 ARG B 4 178.08 -1.87 -0.66 19.01 95 135 26 SER B, 200069 THR B, 200024 ARG B, 200070 ASP B 3.8 179.43 -2.28 -0.76 26.69 92 136 26 SER B, 200024 ARG B, 200070 ASP B 3.81 180.1 -2.8 -0.75 35.03 84 137 200024 ARG B, 200070 ASP B, 26 SER B 3.1 182.18 -2.93 -0.81 34.46 95 138 200024 ARG B, 200070 ASP B, 26 SER B, 3 LEU B 2.43 184.29 -1.25 -0.32 25.88 85 139 200070 ASP B, 26 SER B, 3 LEU B 2.3 186.56 -0.17 -0.29 17.17 85 140 200070 ASP B, 3 LEU B 2.39 189.5 0.15 0.05 24.92 70 141 200070 ASP B, 3 LEU B, 1 ASP B 2.88 191.43 -0.03 -0.23 17.74 70 142 200070 ASP B, 3 LEU B, 1 ASP B, 200072 THR B 3.93 191.57 -0.2 -0.37 13.72 82 143 200070 ASP B, 3 LEU B, 200072 THR B, 1 ASP B 3.95 192.42 -0.98 -0.43 25.3 83 144 200070 ASP B, 3 LEU B, 200072 THR B, 1 ASP B, 63 LYS A 3.91 192.68 -1.56 -0.42 30.14 81 145 3 LEU B, 200072 THR B, 1 ASP B, 63 LYS A 3.8 194.21 -1.08 -0.27 25.25 79 146 3 LEU B, 1 ASP B, 63 LYS A 3.75 195.61 -1.2 -0.1 33.11 70 147 3 LEU B, 1 ASP B, 63 LYS A, 97 THR B 3.75 197.23 -1.08 -0.27 25.25 79 148 3 LEU B, 63 LYS A, 97 THR B, 2 ILE B 3.85 197.71 -0.3 -0.21 13.67 77 149 3 LEU B, 63 LYS A, 97 THR B, 98 PHE B 3.93 198.13 -0.3 -0.21 13.67 77 150 3 LEU B, 63 LYS A, 97 THR B, 98 PHE B, 61 ASN A 4.05 198.7 -0.94 -0.32 11.61 84 151 63 LYS A, 97 THR B, 98 PHE B, 61 ASN A, 46 GLU A 4.37 198.92 -2.4 -0.78 21.56 90 152 97 THR B, 98 PHE B, 61 ASN A, 46 GLU A 4.58 199.18 -2.03 -0.87 14.58 96 153 63 LYS A, 97 THR B, 98 PHE B, 61 ASN A, 46 GLU A 4.98 199.76 -2.4 -0.78 21.56 90 154 3 LEU B, 63 LYS A, 98 PHE B, 61 ASN A, 46 GLU A 5.15 200.8 -1.5 -0.4 21.26 76 155 3 LEU B, 63 LYS A, 98 PHE B, 46 GLU A 5.27 202.09 -1 -0.3 25.73 67 156 3 LEU B, 63 LYS A, 46 GLU A, 64 ILE A 5.23 205.02 0.23 0.35 24.92 76 157 3 LEU B, 63 LYS A, 46 GLU A, 200020 SER B 5.05 205.4 -1.1 -0.35 25.3 80 158 63 LYS A, 46 GLU A, 64 ILE A, 200020 SER B 4.79 206.13 -0.93 -0.18 25.3 92 159 63 LYS A, 46 GLU A, 64 ILE A 2.96 210.7 -0.97 0.09 33.18 84 160 46 GLU A, 64 ILE A, 63 LYS A 2.8 211.61 0.2 -0.04 17.8 90 161 46 GLU A, 64 ILE A, 63 LYS A, 200018 ARG B 2.7 214.49 -0.98 -0.14 26.35 87 162 46 GLU A, 64 ILE A, 200018 ARG B, 40 ARG A 3 217.92 -2 -0.04 38.51 86 163 46 GLU A, 200018 ARG B, 40 ARG A 3.1 220.57 -4.17 -0.66 51.3 81 164 46 GLU A, 64 ILE A, 40 ARG A 2.42 221.23 -1.17 0.08 34.01 87 165 46 GLU A, 40 ARG A 1.11 223.89 -4 -0.78 50.95 80 166 40 ARG A -0.01 230.82 -4.5 -0.42 52 83 167 40 ARG A, 88 SER A 0.2 231.57 -2.45 -0.61 27.69 83 168 40 ARG A, 88 SER A, 91 SER A 0.55 231.93 -1.77 -0.67 19.59 83 169 40 ARG A, 88 SER A, 41 PRO A, 91 SER A 1.01 232.2 -1.83 -0.57 14.66 86 170 40 ARG A, 88 SER A, 91 SER A, 41 PRO A 1.55 233.02 -1.73 -0.53 15.09 70 171 40 ARG A, 88 SER A, 41 PRO A 2.69 235.86 -2.17 -0.44 18.99 70 172 40 ARG A, 41 PRO A, 88 SER A 4.4 237.41 -2.3 -0.49 18.42 86 173 41 PRO A, 88 SER A 4.92 238.93 -1.2 -0.53 1.63 87 174 41 PRO A, 91 SER A, 88 SER A 4.82 239.47 -1.07 -0.68 1.64 97 175 41 PRO A, 91 SER A, 88 SER A, 175 LEU A 4.71 239.93 0.15 -0.22 1.26 86 176 41 PRO A, 88 SER A, 175 LEU A, 180 TYR A 4.58 240.27 0.02 0.3 1.25 69 177 91 SER A, 88 SER A, 175 LEU A, 180 TYR A 4.34 240.71 0.23 0.08 1.27 84 178 91 SER A, 88 SER A, 180 TYR A 4.23 240.8 -0.97 -0.28 1.65 94 179 91 SER A, 88 SER A 4.15 240.98 -0.8 -0.97 1.67 117 180 91 SER A, 88 SER A, 180 TYR A 4.1 241.48 -0.97 -0.28 1.65 94 181 41 PRO A, 88 SER A, 175 LEU A, 180 TYR A 4.08 242.33 0.02 0.3 1.25 69 182 41 PRO A, 91 SER A, 175 LEU A, 180 TYR A 4.09 243.39 0.02 0.3 1.25 69 183 41 PRO A, 175 LEU A, 180 TYR A 4.13 246.68 0.3 0.72 1.11 54 184 41 PRO A, 175 LEU A, 180 TYR A, 173 ALA A 4.39 247.05 0.13 0.34 1.68 54 185 41 PRO A, 175 LEU A, 180 TYR A 4.43 247.75 0.3 0.72 1.11 54 186 41 PRO A, 175 LEU A, 180 TYR A, 173 ALA A 3.87 248.71 0.13 0.34 1.68 54 187 41 PRO A, 180 TYR A, 173 ALA A 3.49 250.68 -1.1 0.07 2.19 54 188 41 PRO A, 180 TYR A, 173 ALA A, 153 GLU A 3.36 252.72 -1.7 -0.23 14.12 61 189 41 PRO A, 173 ALA A, 153 GLU A, 172 PRO A 3.4 254.75 -1.78 -0.53 14.11 64 190 41 PRO A, 153 GLU A, 172 PRO A, 41 ASN B 3.27 255.4 -2.55 -0.52 14.11 74 191 41 PRO A, 153 GLU A, 172 PRO A, 41 ASN B 2.99 256.1 -2.55 -0.52 14.11 74 192 153 GLU A, 172 PRO A, 41 ASN B 2.83 256.58 -2.87 -0.67 18.29 79 193 173 ALA A, 153 GLU A, 172 PRO A, 41 ASN B 2.26 259.89 -2.25 -0.7 14.56 79 194 173 ALA A, 153 GLU A, 41 ASN B, 172 PRO A 2.25 260.46 -1.95 -0.88 15.01 90 195 173 ALA A, 153 GLU A, 41 ASN B, 172 PRO A, 182 LEU A 2.27 260.86 -0.8 -0.47 12.03 78 196 153 GLU A, 41 ASN B, 172 PRO A, 182 LEU A, 171 PHE A 2.33 261.15 -0.8 -0.47 12.03 78 197 41 ASN B, 172 PRO A, 182 LEU A, 171 PHE A 2.48 262.05 -0.13 -0.31 2.57 79 198 41 ASN B, 182 LEU A, 171 PHE A 2.87 262.27 -0.03 -0.14 2.3 79 199 41 ASN B, 182 LEU A, 170 THR A 2.81 262.56 -0.13 -0.13 1.72 88 200 41 ASN B, 182 LEU A, 170 THR A, 155 VAL A 2.5 264.37 -0.2 -0.3 2.14 88 201 41 ASN B, 170 THR A, 155 VAL A 2.5 267.67 -1.53 -0.78 2.81 105 202 41 ASN B, 170 THR A, 155 VAL A, 156 THR A, 157 VAL A 2.69 267.92 -1.14 -0.78 2.69 106 203 41 ASN B, 156 THR A, 157 VAL A 2.78 268.06 -1.53 -0.78 2.81 105 204 41 ASN B, 170 THR A, 156 THR A, 157 VAL A 2.97 268.4 -1.33 -0.78 2.52 106 205 41 ASN B, 170 THR A, 155 VAL A 3.15 268.58 -1.53 -0.78 2.81 105 206 41 ASN B, 170 THR A, 157 VAL A 3.34 268.81 -1.53 -0.78 2.81 105 207 41 ASN B, 170 THR A, 156 THR A, 157 VAL A 3.14 269.19 -1.33 -0.78 2.52 106 208 41 ASN B, 170 THR A, 157 VAL A 3.16 272.3 -1.53 -0.78 2.81 105 209 41 ASN B, 170 THR A, 157 VAL A, 40 THR B 4.21 273.16 -1.25 -0.79 2.95 105 210 41 ASN B, 170 THR A, 157 VAL A, 40 THR B, 168 VAL A 4.55 273.31 -1.08 -0.79 3.04 105 211 170 THR A, 157 VAL A, 40 THR B, 168 VAL A 4.61 273.46 0.68 -0.31 2.14 102 212 170 THR A, 157 VAL A, 40 THR B, 168 VAL A, 169 LYS B 4.58 273.6 -1.16 -0.72 12.26 89 213 157 VAL A, 40 THR B, 168 VAL A, 169 LYS B 4.59 273.65 -0.13 -0.22 14.1 85 214 170 THR A, 157 VAL A, 168 VAL A, 169 LYS B 4.38 274.45 -0.2 -0.21 13.67 92 215 157 VAL A, 168 VAL A, 169 LYS B, 165 SER A 4.21 274.89 -0.13 -0.22 14.1 85 216 168 VAL A, 169 LYS B, 165 SER A 3.01 278.94 -0.03 -0.03 17.67 85 217 168 VAL A, 169 LYS B, 165 SER A 1.72 281.05 -1.57 -0.67 18.75 72 218 168 VAL A, 169 LYS B, 165 SER A, 167 GLY A 1.35 282.39 -1.28 -0.7 14.91 72 219 168 VAL A, 169 LYS B, 167 GLY A 1.5 283.29 -1.57 -0.67 18.75 72 220 168 VAL A, 169 LYS B, 167 GLY A, 169 HIS A 1.73 283.54 -1.98 -0.44 26.97 81 221 168 VAL A, 169 LYS B, 167 GLY A, 169 HIS A 1.84 283.77 -1.98 -0.44 26.97 81 222 168 VAL A, 169 LYS B, 167 GLY A, 169 HIS A 2.08 283.83 -1.98 -0.44 26.97 81 223 168 VAL A, 169 LYS B, 167 GLY A, 169 HIS A 2.06 284.3 -1.98 -0.44 26.97 81 224 169 LYS B, 167 GLY A, 169 HIS A, 167 ASP B 1.97 284.99 -2.75 -0.5 38.55 83 225 169 LYS B, 167 GLY A, 167 ASP B 1.85 286.43 -2.6 -0.75 34.19 79 226 169 LYS B, 167 GLY A 1.79 287.2 -2.15 -0.61 26.44 72 227 169 LYS B, 167 GLY A, 166 SER A 1.7 288.5 -1.57 -0.67 18.75 72 228 169 LYS B, 167 GLY A, 166 SER A 1.72 291.99 -1.7 -0.73 18.18 94 229 169 LYS B, 167 GLY A, 166 SER A, 170 ASP B 3.26 293.36 -2.15 -0.81 26.06 91 230 167 GLY A, 166 SER A, 170 ASP B, 169 LYS B 4.76 293.85 -1.28 -0.9 14.53 101 231 167 GLY A, 166 SER A, 170 ASP B 4.88 294.75 -1.57 -0.94 18.25 101 232 167 GLY A, 166 SER A, 170 ASP B, 140 MET A 4.88 295.81 -0.7 -0.45 14.05 98 233 167 GLY A, 170 ASP B, 140 MET A 4.48 297.52 -0.67 -0.28 18.17 89 234 167 GLY A, 170 ASP B, 140 MET A, 138 ASN B 4.19 298.81 -1.38 -0.4 14.47 94 235 170 ASP B, 140 MET A, 138 ASN B 3.87 300.2 -1.7 -0.27 18.17 94 236 140 MET A, 138 ASN B, 187 THR A 3.47 301.99 -0.77 -0.18 2.16 101 237 140 MET A, 138 ASN B, 187 THR A, 114 THR B 2.96 303.55 -0.75 -0.33 2.03 102 238 140 MET A, 187 THR A, 114 THR B 2.65 305.3 0.17 -0.18 1.58 102 239 140 MET A, 114 THR B 2.47 306.84 0.6 0.12 1.55 100 240 140 MET A, 114 THR B, 138 ASN A 2.4 307.32 -0.77 -0.18 2.16 101 241 140 MET A, 114 THR B, 138 ASN A, 139 SER A 2.33 308.31 -0.78 -0.38 2.04 105 242 140 MET A, 114 THR B, 138 ASN A 2.37 311.11 -0.77 -0.18 2.16 101 243 114 THR B, 138 ASN A 2.85 314.54 -2.1 -0.77 2.52 105 244 114 THR B, 138 ASN A, 207 LYS B 3.27 316.82 -2.7 -0.65 18.18 94 245 114 THR B, 138 ASN A, 207 LYS B, 115 VAL B 3.44 317.92 -2.13 -0.69 14.48 94 246 114 THR B, 138 ASN A, 207 LYS B, 136 GLN A 3.4 318.87 -2.13 -0.69 14.48 94 247 114 THR B, 138 ASN A, 207 LYS B, 115 VAL B, 136 GLN A 2.99 320 -1.78 -0.71 12.26 94 248 114 THR B, 207 LYS B, 115 VAL B, 136 GLN A 2.68 320.45 -1.35 -0.7 14.48 89 249 114 THR B, 138 ASN A, 207 LYS B, 115 VAL B, 136 GLN A 2.63 320.76 -1.78 -0.71 12.26 94 250 114 THR B, 207 LYS B, 115 VAL B, 136 GLN A, 116 SER B 2.59 321.12 -1.24 -0.75 11.92 98 251 114 THR B, 115 VAL B, 136 GLN A, 116 SER B, 140 MET A 2.57 322.66 -0.54 -0.83 2.69 112 252 207 LYS B, 115 VAL B, 136 GLN A, 116 SER B 2.59 323.35 -1.38 -0.75 14.48 94 253 115 VAL B, 136 GLN A, 116 SER B 2.59 324.56 -0.53 -0.86 2.81 117 254 115 VAL B, 136 GLN A, 116 SER B, 135 ALA A 2.54 325.27 0.05 -0.64 2.11 108 255 115 VAL B, 116 SER B, 135 ALA A 2.26 326.48 0.2 -0.58 1.68 108 256 116 SER B, 135 ALA A, 117 ILE B 1.98 328.95 0.2 -0.58 1.68 108 257 135 ALA A, 117 ILE B, 116 SER B 1.94 329.45 0.33 -0.53 2.25 100 258 135 ALA A, 116 SER B, 117 ILE B 1.91 329.95 1.97 0.34 1.17 101 259 135 ALA A, 117 ILE B 1.87 331.64 3.15 0.92 0.07 101 260 135 ALA A, 117 ILE B, 208 SER B 1.87 332.2 1.97 0.34 1.17 101 261 135 ALA A, 117 ILE B, 208 SER B, 132 GLY A 1.87 332.68 1.38 0.06 1.72 101 262 135 ALA A, 117 ILE B, 132 GLY A 1.86 333.28 1.97 0.34 1.17 101 263 117 ILE B, 208 SER B, 132 GLY A 1.85 333.48 1.23 0.07 2.3 103 264 117 ILE B, 132 GLY A, 209 PHE B 1.63 334.61 2.3 0.79 1.29 77 265 117 ILE B, 132 GLY A, 209 PHE B, 119 PRO B 1.5 335.1 0.1 -0.09 2.17 54 266 117 ILE B, 132 GLY A, 119 PRO B 1.48 335.38 -0.8 -0.56 2.78 58 267 117 ILE B, 132 GLY A, 119 PRO B 1.52 335.76 0.83 0.31 1.7 80 268 117 ILE B, 132 GLY A, 209 PHE B, 119 PRO B 1.65 336.41 0.1 -0.09 2.17 54 269 132 GLY A, 209 PHE B, 119 PRO B 1.74 340 0.27 0.15 1.77 54 270 132 GLY A, 209 PHE B, 119 PRO B, 210 ASN B 2.11 340.49 0.1 -0.09 2.17 54 271 132 GLY A, 209 PHE B, 119 PRO B, 210 ASN B, 133 SER A 2.19 341.04 0 -0.23 2.41 54 272 132 GLY A, 119 PRO B, 210 ASN B, 133 SER A, 218 ARG A 2.29 342.02 -1.46 -0.58 12.74 70 273 132 GLY A, 119 PRO B, 210 ASN B, 218 ARG A 2.26 342.29 -1.73 -0.53 15.09 70 274 119 PRO B, 210 ASN B, 218 ARG A 2.04 343.62 -2.17 -0.44 18.99 70 275 209 PHE B, 119 PRO B, 210 ASN B, 218 ARG A 2.01 344.12 -0.93 0.01 14.33 64 276 119 PRO B, 210 ASN B, 218 ARG A, 211 ARG B 2.01 344.76 -1.73 -0.53 15.09 70 277 119 PRO B, 210 ASN B, 218 ARG A, 211 ARG B, 186 TYR B 2.08 345.11 -1.64 -0.2 12.39 63 278 119 PRO B, 218 ARG A, 211 ARG B, 186 TYR B 2.16 346.81 -1.95 -0.05 14.64 63 279 218 ARG A, 186 TYR B, 211 ARG B 2.31 348.42 -3.43 0.09 35.2 72 280 218 ARG A, 186 TYR B, 211 ARG B, 125 LEU B 2.41 348.42 -1.63 0.35 26.44 67 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
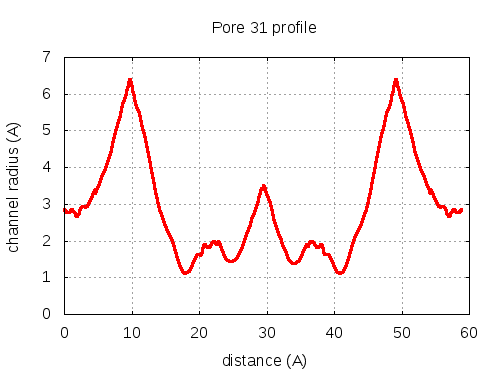
Unique lining residues set - all
100101 THR C, 300106 VAL C, 300107 THR C, 300110 LEU C, 300103 PHE C, 100100 ILE C, 100100 ILE C, 100104 GLY C, 100103 PHE C, 200100 ILE C, 100107 THR C, 100103 PHE C, 200101 THR C, 100106 VAL C, 200100 ILE C, 100110 LEU C, 200101 THR C
Unique lining residues set - sidechains
300106 VAL C, 300107 THR C, 300110 LEU C, 100100 ILE C, 100103 PHE C, 200100 ILE C, 100107 THR C, 100106 VAL C, 100110 LEU C, 200101 THR C
Physicochemical properties of lining side-chains
Charge: 0 (0-0)
Hydropathy: 1.1
Hydrophobicity: 0.02
Polarity: 1.81
Mutability: 88
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 100101 THR C, 300106 VAL C, 300107 THR C, 300110 LEU C 1.85 0.66 1.73 0.18 1.33 86 2 100101 THR C, 300106 VAL C, 300107 THR C 1.83 0.94 1.03 -0.15 1.72 102 3 100101 THR C, 300106 VAL C, 300107 THR C, 300103 PHE C 1.85 1.53 0.68 -0.31 2.14 102 4 100101 THR C, 300106 VAL C, 300107 THR C, 300103 PHE C, 100100 ILE C 1.96 1.68 0.46 -0.41 2.39 102 5 100101 THR C, 300107 THR C, 300103 PHE C, 100100 ILE C 1.94 1.79 -0.48 -0.79 2.95 107 6 100101 THR C, 300107 THR C, 300103 PHE C, 100100 ILE C 1.91 2.57 0.75 -0.14 2.14 105 7 300107 THR C, 300103 PHE C, 100100 ILE C 1.52 4.16 1.13 0.08 1.72 105 8 300107 THR C, 300103 PHE C, 100100 ILE C, 100104 GLY C 1.44 5.18 0.75 -0.14 2.14 105 9 300107 THR C, 100100 ILE C, 100104 GLY C 1.65 8.26 1.13 0.08 1.72 105 10 100100 ILE C, 100104 GLY C, 100103 PHE C 3.3 8.97 2.3 0.79 1.29 77 11 100100 ILE C, 100104 GLY C, 100103 PHE C, 200100 ILE C 3.42 9.17 1.63 0.39 1.81 77 12 100104 GLY C, 100103 PHE C, 200100 ILE C 3.31 9.4 2.3 0.79 1.29 77 13 100104 GLY C, 100103 PHE C, 200100 ILE C, 100107 THR C 2.74 10.42 1.55 0.4 1.38 87 14 100103 PHE C, 200100 ILE C, 100107 THR C 1.39 14.66 2.2 0.8 0.71 87 15 200100 ILE C, 100107 THR C, 100103 PHE C 1.7 15.07 1.13 0.08 1.72 105 16 200100 ILE C, 100107 THR C, 100103 PHE C, 200101 THR C 1.86 15.74 0.75 -0.14 2.14 105 17 100107 THR C, 100103 PHE C, 200101 THR C, 100106 VAL C, 200100 ILE C 1.98 15.91 0.46 -0.41 2.39 102 18 100107 THR C, 100103 PHE C, 200101 THR C, 100106 VAL C 1.87 16.74 0.68 -0.31 2.14 102 19 100107 THR C, 200101 THR C, 100106 VAL C 1.84 17.05 1.03 -0.15 1.72 102 20 100107 THR C, 200101 THR C, 100106 VAL C, 100110 LEU C 1.77 17.81 1.73 0.18 1.33 86 21 100106 VAL C, 100110 LEU C, 200101 THR C 1.22 22.08 2.43 0.5 0.64 86 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
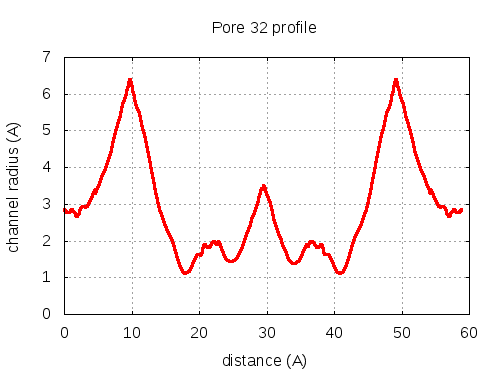
Unique lining residues set - all
100106 VAL C, 100107 THR C, 100110 LEU C, 200101 THR C, 100103 PHE C, 200100 ILE C, 200100 ILE C, 200104 GLY C, 200103 PHE C, 100 ILE C, 200107 THR C, 200103 PHE C, 101 THR C, 100 ILE C, 200106 VAL C, 200110 LEU C, 101 THR C
Unique lining residues set - sidechains
100106 VAL C, 100107 THR C, 100110 LEU C, 200100 ILE C, 200103 PHE C, 100 ILE C, 200107 THR C, 200106 VAL C, 200110 LEU C, 101 THR C
Physicochemical properties of lining side-chains
Charge: 0 (0-0)
Hydropathy: 1.1
Hydrophobicity: 0.02
Polarity: 1.81
Mutability: 88
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 100106 VAL C, 100107 THR C, 100110 LEU C, 200101 THR C 1.85 0.65 1.73 0.18 1.33 86 2 100106 VAL C, 100107 THR C, 200101 THR C 1.83 0.94 1.03 -0.15 1.72 102 3 100106 VAL C, 100107 THR C, 200101 THR C, 100103 PHE C 1.85 1.53 0.68 -0.31 2.14 102 4 100106 VAL C, 100107 THR C, 200101 THR C, 100103 PHE C, 200100 ILE C 1.96 1.68 0.46 -0.41 2.39 102 5 100107 THR C, 200101 THR C, 100103 PHE C, 200100 ILE C 1.94 1.79 -0.48 -0.79 2.95 107 6 100107 THR C, 200101 THR C, 100103 PHE C, 200100 ILE C 1.91 2.57 0.75 -0.14 2.14 105 7 100107 THR C, 100103 PHE C, 200100 ILE C 1.52 4.16 1.13 0.08 1.72 105 8 100107 THR C, 100103 PHE C, 200100 ILE C, 200104 GLY C 1.44 5.18 0.75 -0.14 2.14 105 9 100107 THR C, 200100 ILE C, 200104 GLY C 1.65 8.26 1.13 0.08 1.72 105 10 200100 ILE C, 200104 GLY C, 200103 PHE C 3.3 8.97 2.3 0.79 1.29 77 11 200100 ILE C, 200104 GLY C, 200103 PHE C, 100 ILE C 3.42 9.17 1.63 0.39 1.81 77 12 200104 GLY C, 200103 PHE C, 100 ILE C 3.31 9.4 2.3 0.79 1.29 77 13 200104 GLY C, 200103 PHE C, 100 ILE C, 200107 THR C 2.74 10.42 1.55 0.4 1.38 87 14 200103 PHE C, 100 ILE C, 200107 THR C 1.39 14.66 2.2 0.8 0.71 87 15 100 ILE C, 200107 THR C, 200103 PHE C 1.7 15.07 1.13 0.08 1.72 105 16 100 ILE C, 200107 THR C, 200103 PHE C, 101 THR C 1.86 15.74 0.75 -0.14 2.14 105 17 200107 THR C, 200103 PHE C, 101 THR C, 100 ILE C, 200106 VAL C 1.98 15.91 0.46 -0.41 2.39 102 18 200107 THR C, 200103 PHE C, 101 THR C, 200106 VAL C 1.87 16.74 0.68 -0.31 2.14 102 19 200107 THR C, 101 THR C, 200106 VAL C 1.84 17.05 1.03 -0.15 1.72 102 20 200107 THR C, 101 THR C, 200106 VAL C, 200110 LEU C 1.77 17.81 1.73 0.18 1.33 86 21 200106 VAL C, 200110 LEU C, 101 THR C 1.22 22.08 2.43 0.5 0.64 86 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
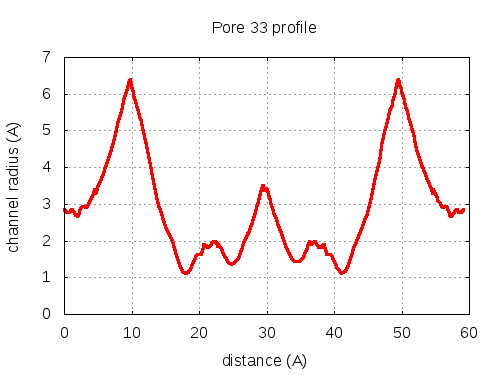
Unique lining residues set - all
106 VAL C, 107 THR C, 110 LEU C, 300101 THR C, 103 PHE C, 300100 ILE C, 300100 ILE C, 103 PHE C, 104 GLY C, 100 ILE C, 100 ILE C, 200107 THR C, 200103 PHE C, 101 THR C, 200106 VAL C, 200110 LEU C, 101 THR C
Unique lining residues set - sidechains
106 VAL C, 107 THR C, 110 LEU C, 300100 ILE C, 103 PHE C, 100 ILE C, 200107 THR C, 200106 VAL C, 200110 LEU C, 101 THR C
Physicochemical properties of lining side-chains
Charge: 0 (0-0)
Hydropathy: 1.2
Hydrophobicity: 0.05
Polarity: 1.75
Mutability: 88
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 106 VAL C, 107 THR C, 110 LEU C, 300101 THR C 1.86 0.64 1.73 0.18 1.33 86 2 106 VAL C, 107 THR C, 300101 THR C 1.84 0.92 1.03 -0.15 1.72 102 3 106 VAL C, 107 THR C, 300101 THR C, 103 PHE C 1.86 1.52 0.68 -0.31 2.14 102 4 106 VAL C, 107 THR C, 300101 THR C, 103 PHE C, 300100 ILE C 1.97 1.79 0.46 -0.41 2.39 102 5 107 THR C, 300101 THR C, 103 PHE C, 300100 ILE C 1.84 2.45 0.75 -0.14 2.14 105 6 107 THR C, 103 PHE C, 300100 ILE C 1.55 3.85 1.13 0.08 1.72 105 7 107 THR C, 300100 ILE C, 103 PHE C 1.38 6.59 2.2 0.8 0.71 87 8 107 THR C, 103 PHE C, 104 GLY C 2.25 7.88 0.57 -0.07 1.8 79 9 300100 ILE C, 103 PHE C, 104 GLY C 3.01 8.32 2.3 0.79 1.29 77 10 300100 ILE C, 103 PHE C, 104 GLY C, 100 ILE C 3.31 8.63 1.63 0.39 1.81 77 11 103 PHE C, 104 GLY C, 100 ILE C 3.27 9.73 2.3 0.79 1.29 77 12 104 GLY C, 100 ILE C, 200107 THR C 1.59 13.63 1.13 0.08 1.72 105 13 100 ILE C, 200107 THR C, 200103 PHE C 1.43 15.48 1.13 0.08 1.72 105 14 100 ILE C, 200107 THR C, 200103 PHE C, 101 THR C 1.91 16.09 0.75 -0.14 2.14 105 15 100 ILE C, 200107 THR C, 200103 PHE C, 101 THR C, 200106 VAL C 1.96 16.26 0.46 -0.41 2.39 102 16 200107 THR C, 200103 PHE C, 101 THR C, 200106 VAL C 1.85 17.1 0.68 -0.31 2.14 102 17 200107 THR C, 101 THR C, 200106 VAL C 1.83 17.4 1.03 -0.15 1.72 102 18 200107 THR C, 101 THR C, 200106 VAL C, 200110 LEU C 1.75 18.15 1.73 0.18 1.33 86 19 200106 VAL C, 200110 LEU C, 101 THR C 1.22 22.4 2.43 0.5 0.64 86 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
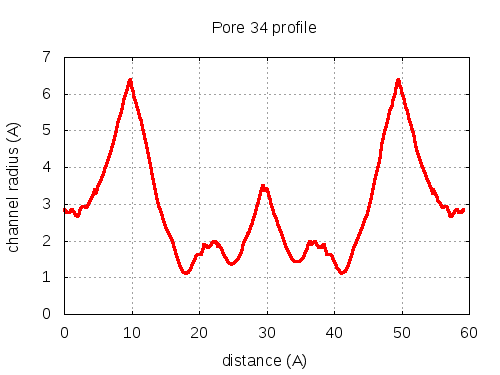
Unique lining residues set - all
100101 THR C, 300106 VAL C, 300107 THR C, 300110 LEU C, 300103 PHE C, 100100 ILE C, 100100 ILE C, 300103 PHE C, 300104 GLY C, 300100 ILE C, 300100 ILE C, 107 THR C, 103 PHE C, 300101 THR C, 106 VAL C, 110 LEU C, 300101 THR C
Unique lining residues set - sidechains
300106 VAL C, 300107 THR C, 300110 LEU C, 100100 ILE C, 300103 PHE C, 300100 ILE C, 107 THR C, 106 VAL C, 110 LEU C, 300101 THR C
Physicochemical properties of lining side-chains
Charge: 0 (0-0)
Hydropathy: 1.2
Hydrophobicity: 0.05
Polarity: 1.75
Mutability: 88
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 100101 THR C, 300106 VAL C, 300107 THR C, 300110 LEU C 1.86 0.64 1.73 0.18 1.33 86 2 100101 THR C, 300106 VAL C, 300107 THR C 1.84 0.92 1.03 -0.15 1.72 102 3 100101 THR C, 300106 VAL C, 300107 THR C, 300103 PHE C 1.86 1.52 0.68 -0.31 2.14 102 4 100101 THR C, 300106 VAL C, 300107 THR C, 300103 PHE C, 100100 ILE C 1.97 1.79 0.46 -0.41 2.39 102 5 100101 THR C, 300107 THR C, 300103 PHE C, 100100 ILE C 1.84 2.45 0.75 -0.14 2.14 105 6 300107 THR C, 300103 PHE C, 100100 ILE C 1.55 3.85 1.13 0.08 1.72 105 7 300107 THR C, 100100 ILE C, 300103 PHE C 1.38 6.59 2.2 0.8 0.71 87 8 300107 THR C, 300103 PHE C, 300104 GLY C 2.25 7.88 0.57 -0.07 1.8 79 9 100100 ILE C, 300103 PHE C, 300104 GLY C 3 8.32 2.3 0.79 1.29 77 10 100100 ILE C, 300103 PHE C, 300104 GLY C, 300100 ILE C 3.31 8.63 1.63 0.39 1.81 77 11 300103 PHE C, 300104 GLY C, 300100 ILE C 3.27 9.73 2.3 0.79 1.29 77 12 300104 GLY C, 300100 ILE C, 107 THR C 1.59 13.63 1.13 0.08 1.72 105 13 300100 ILE C, 107 THR C, 103 PHE C 1.43 15.48 1.13 0.08 1.72 105 14 300100 ILE C, 107 THR C, 103 PHE C, 300101 THR C 1.91 16.09 0.75 -0.14 2.14 105 15 300100 ILE C, 107 THR C, 103 PHE C, 300101 THR C, 106 VAL C 1.96 16.26 0.46 -0.41 2.39 102 16 107 THR C, 103 PHE C, 300101 THR C, 106 VAL C 1.85 17.1 0.68 -0.31 2.14 102 17 107 THR C, 300101 THR C, 106 VAL C 1.83 17.4 1.03 -0.15 1.72 102 18 107 THR C, 300101 THR C, 106 VAL C, 110 LEU C 1.75 18.15 1.73 0.18 1.33 86 19 106 VAL C, 110 LEU C, 300101 THR C 1.22 22.4 2.43 0.5 0.64 86 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
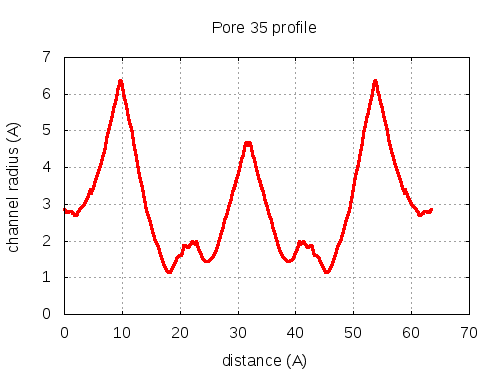
Unique lining residues set - all
100101 THR C, 300106 VAL C, 300107 THR C, 300110 LEU C, 300103 PHE C, 100100 ILE C, 100100 ILE C, 100104 GLY C, 100103 PHE C, 200100 ILE C, 100107 THR C, 200107 THR C, 100 ILE C, 107 THR C, 300100 ILE C, 104 GLY C, 103 PHE C, 200103 PHE C, 101 THR C, 100 ILE C, 200106 VAL C, 200110 LEU C, 101 THR C
Unique lining residues set - sidechains
300106 VAL C, 300107 THR C, 300110 LEU C, 100100 ILE C, 100103 PHE C, 200100 ILE C, 100107 THR C, 200107 THR C, 100 ILE C, 107 THR C, 300100 ILE C, 103 PHE C, 200106 VAL C, 200110 LEU C, 101 THR C
Physicochemical properties of lining side-chains
Charge: 0 (0-0)
Hydropathy: 1.4
Hydrophobicity: 0.2
Polarity: 1.51
Mutability: 90
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 100101 THR C, 300106 VAL C, 300107 THR C, 300110 LEU C 1.92 0.44 1.73 0.18 1.33 86 2 100101 THR C, 300106 VAL C, 300107 THR C 1.84 0.84 1.03 -0.15 1.72 102 3 100101 THR C, 300106 VAL C, 300107 THR C, 300103 PHE C 1.84 1.62 0.68 -0.31 2.14 102 4 100101 THR C, 300106 VAL C, 300107 THR C, 300103 PHE C, 100100 ILE C 1.95 1.79 0.46 -0.41 2.39 102 5 100101 THR C, 300107 THR C, 300103 PHE C, 100100 ILE C 1.91 2.67 0.75 -0.14 2.14 105 6 300107 THR C, 300103 PHE C, 100100 ILE C 1.44 5 1.13 0.08 1.72 105 7 300107 THR C, 100100 ILE C, 100104 GLY C 1.6 8.28 1.13 0.08 1.72 105 8 100100 ILE C, 100104 GLY C, 100103 PHE C 3.32 8.82 2.3 0.79 1.29 77 9 100100 ILE C, 100104 GLY C, 100103 PHE C, 200100 ILE C 3.59 9.28 2.85 1.04 1 85 10 100100 ILE C, 100104 GLY C, 200100 ILE C, 100107 THR C 3.82 9.56 1.98 0.51 1.33 104 11 300107 THR C, 100100 ILE C, 100104 GLY C, 200100 ILE C, 100107 THR C 4.02 10.62 1.44 0.26 1.39 105 12 300107 THR C, 100100 ILE C, 200100 ILE C, 100107 THR C, 200107 THR C 4.66 10.79 1.38 0.26 1.05 105 13 300107 THR C, 200107 THR C, 100 ILE C, 107 THR C, 300100 ILE C 4.61 10.97 1.38 0.26 1.05 105 14 300107 THR C, 100100 ILE C, 200100 ILE C, 100107 THR C, 200107 THR C 4.62 11.18 1.38 0.26 1.05 105 15 100100 ILE C, 200107 THR C, 100 ILE C, 107 THR C, 300100 ILE C 4.66 11.45 2.42 0.78 0.74 104 16 300107 THR C, 200107 THR C, 100 ILE C, 107 THR C, 300100 ILE C 4.53 11.82 1.38 0.26 1.05 105 17 200107 THR C, 100 ILE C, 107 THR C, 300100 ILE C, 104 GLY C 4.09 12.51 1.44 0.26 1.39 105 18 100 ILE C, 107 THR C, 300100 ILE C, 104 GLY C 3.88 12.74 1.98 0.51 1.33 104 19 100 ILE C, 300100 ILE C, 104 GLY C, 103 PHE C 3.55 13.14 2.85 1.04 1 85 20 100 ILE C, 104 GLY C, 103 PHE C 3.17 14.36 2.3 0.79 1.29 77 21 200107 THR C, 100 ILE C, 104 GLY C 1.76 17.77 1.13 0.08 1.72 105 22 200107 THR C, 100 ILE C, 104 GLY C, 200103 PHE C 1.43 18.92 0.75 -0.14 2.14 105 23 200107 THR C, 100 ILE C, 200103 PHE C 1.59 19.61 1.13 0.08 1.72 105 24 200107 THR C, 100 ILE C, 200103 PHE C, 101 THR C 1.9 20.37 0.75 -0.14 2.14 105 25 200107 THR C, 200103 PHE C, 101 THR C, 100 ILE C, 200106 VAL C 1.97 20.63 0.46 -0.41 2.39 102 26 200107 THR C, 200103 PHE C, 101 THR C, 200106 VAL C 1.85 21.43 0.68 -0.31 2.14 102 27 200107 THR C, 101 THR C, 200106 VAL C 1.83 21.79 1.03 -0.15 1.72 102 28 200107 THR C, 101 THR C, 200106 VAL C, 200110 LEU C 1.85 22.22 1.73 0.18 1.33 86 29 200106 VAL C, 200110 LEU C, 101 THR C 1.22 26.63 2.43 0.5 0.64 86 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
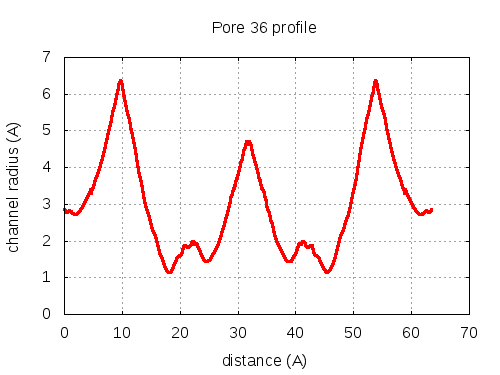
Unique lining residues set - all
106 VAL C, 107 THR C, 110 LEU C, 300101 THR C, 103 PHE C, 300100 ILE C, 300100 ILE C, 300104 GLY C, 300103 PHE C, 100100 ILE C, 300107 THR C, 100107 THR C, 100 ILE C, 200100 ILE C, 200107 THR C, 200104 GLY C, 200103 PHE C, 100103 PHE C, 200101 THR C, 100106 VAL C, 200100 ILE C, 100110 LEU C, 200101 THR C
Unique lining residues set - sidechains
106 VAL C, 107 THR C, 110 LEU C, 300100 ILE C, 300103 PHE C, 100100 ILE C, 300107 THR C, 100107 THR C, 100 ILE C, 200100 ILE C, 200107 THR C, 200103 PHE C, 100106 VAL C, 100110 LEU C, 200101 THR C
Physicochemical properties of lining side-chains
Charge: 0 (0-0)
Hydropathy: 1.3
Hydrophobicity: 0.16
Polarity: 1.54
Mutability: 90
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 106 VAL C, 107 THR C, 110 LEU C, 300101 THR C 1.92 0.48 1.73 0.18 1.33 86 2 106 VAL C, 107 THR C, 300101 THR C 1.83 0.9 1.03 -0.15 1.72 102 3 106 VAL C, 107 THR C, 300101 THR C, 103 PHE C 1.85 1.48 0.68 -0.31 2.14 102 4 106 VAL C, 107 THR C, 300101 THR C, 103 PHE C, 300100 ILE C 1.97 1.7 0.46 -0.41 2.39 102 5 107 THR C, 300101 THR C, 103 PHE C, 300100 ILE C 1.94 1.84 -0.48 -0.79 2.95 107 6 107 THR C, 300101 THR C, 103 PHE C, 300100 ILE C 1.91 2.49 0.75 -0.14 2.14 105 7 107 THR C, 103 PHE C, 300100 ILE C 1.47 4.89 1.13 0.08 1.72 105 8 107 THR C, 103 PHE C, 300100 ILE C, 300104 GLY C 1.56 6.29 0.75 -0.14 2.14 105 9 107 THR C, 300100 ILE C, 300104 GLY C 2.19 8.34 1.13 0.08 1.72 105 10 300100 ILE C, 300104 GLY C, 300103 PHE C 3.36 8.82 2.3 0.79 1.29 77 11 300100 ILE C, 300104 GLY C, 300103 PHE C, 100100 ILE C 3.59 9.34 2.85 1.04 1 85 12 300100 ILE C, 300104 GLY C, 100100 ILE C, 300107 THR C 3.87 9.68 1.98 0.51 1.33 104 13 107 THR C, 300100 ILE C, 300104 GLY C, 100100 ILE C, 300107 THR C 4.09 10.48 1.44 0.26 1.39 105 14 107 THR C, 300100 ILE C, 100100 ILE C, 300107 THR C, 100107 THR C 4.57 10.74 1.38 0.26 1.05 105 15 107 THR C, 100107 THR C, 100 ILE C, 200100 ILE C, 200107 THR C 4.62 10.92 1.38 0.26 1.05 105 16 107 THR C, 300100 ILE C, 100100 ILE C, 300107 THR C, 100107 THR C 4.68 10.98 1.38 0.26 1.05 105 17 107 THR C, 100107 THR C, 100 ILE C, 200100 ILE C, 200107 THR C 4.68 11.04 1.38 0.26 1.05 105 18 107 THR C, 100100 ILE C, 100107 THR C, 100 ILE C, 200107 THR C 4.69 11.11 1.38 0.26 1.05 105 19 107 THR C, 300100 ILE C, 100100 ILE C, 300107 THR C, 100107 THR C 4.62 11.52 1.38 0.26 1.05 105 20 107 THR C, 100107 THR C, 100 ILE C, 200100 ILE C, 200107 THR C 4.54 11.92 1.38 0.26 1.05 105 21 100107 THR C, 100 ILE C, 200100 ILE C, 200107 THR C, 200104 GLY C 4.06 12.65 1.44 0.26 1.39 105 22 100 ILE C, 200100 ILE C, 200107 THR C, 200104 GLY C 3.84 12.88 1.98 0.51 1.33 104 23 100 ILE C, 200100 ILE C, 200104 GLY C, 200103 PHE C 3.58 13.2 2.85 1.04 1 85 24 200100 ILE C, 200104 GLY C, 200103 PHE C 3.33 14.05 2.3 0.79 1.29 77 25 100107 THR C, 200100 ILE C, 200104 GLY C 2.07 17.29 1.13 0.08 1.72 105 26 100107 THR C, 200100 ILE C, 200104 GLY C, 100103 PHE C 1.51 18.65 0.75 -0.14 2.14 105 27 100107 THR C, 200100 ILE C, 100103 PHE C 1.5 19.56 1.13 0.08 1.72 105 28 100107 THR C, 200100 ILE C, 100103 PHE C, 200101 THR C 1.84 20.48 0.75 -0.14 2.14 105 29 100107 THR C, 100103 PHE C, 200101 THR C, 100106 VAL C, 200100 ILE C 1.97 20.78 0.46 -0.41 2.39 102 30 100107 THR C, 100103 PHE C, 200101 THR C, 100106 VAL C 1.83 21.66 0.68 -0.31 2.14 102 31 100107 THR C, 200101 THR C, 100106 VAL C, 100110 LEU C 1.78 22.51 1.73 0.18 1.33 86 32 100106 VAL C, 100110 LEU C, 200101 THR C 1.25 26.7 2.43 0.5 0.64 86 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
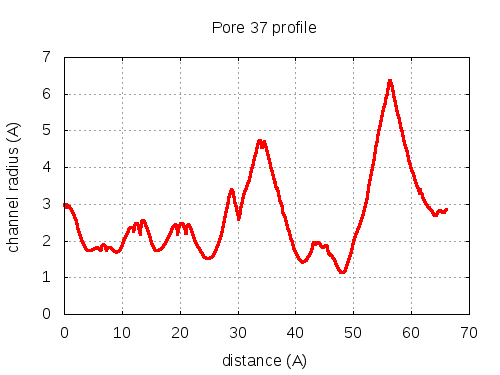
Unique lining residues set - all
119 GLN C, 100119 GLN C, 200115 VAL C, 300115 VAL C, 300119 GLN C, 115 VAL C, 100115 VAL C, 200119 GLN C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 300104 GLY C, 300100 ILE C, 100 ILE C, 100100 ILE C, 200100 ILE C, 300103 PHE C, 103 PHE C, 300101 THR C, 300100 ILE C, 106 VAL C, 110 LEU C, 300101 THR C
Unique lining residues set - sidechains
119 GLN C, 100119 GLN C, 200115 VAL C, 300115 VAL C, 300119 GLN C, 115 VAL C, 100115 VAL C, 200119 GLN C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 300100 ILE C, 100 ILE C, 100100 ILE C, 200100 ILE C, 300103 PHE C, 106 VAL C, 110 LEU C, 300101 THR C
Physicochemical properties of lining side-chains
Charge: 0 (0-0)
Hydropathy: 1.4
Hydrophobicity: 0.12
Polarity: 1.16
Mutability: 94
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 119 GLN C, 100119 GLN C, 200115 VAL C, 300115 VAL C, 300119 GLN C 3.01 0.07 -0.42 -0.21 2.17 89 2 200115 VAL C, 300115 VAL C, 300119 GLN C, 115 VAL C, 100115 VAL C 2.91 0.42 2.66 0.68 0.81 95 3 119 GLN C, 100119 GLN C, 300119 GLN C, 200119 GLN C 2.9 1.42 -3.5 -1.1 3.53 84 4 119 GLN C, 200115 VAL C, 300115 VAL C, 115 VAL C, 100115 VAL C 2.4 3.1 2.66 0.68 0.81 95 5 200115 VAL C, 300115 VAL C, 115 VAL C, 100115 VAL C 1.71 10.88 4.2 1.13 0.13 98 6 200115 VAL C, 300115 VAL C, 115 VAL C, 100115 VAL C, 100111 ALA C 2.29 11.46 3.72 0.91 0.1 98 7 115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.26 12.12 2.28 0.24 0.03 99 8 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.37 12.64 2.28 0.24 0.03 99 9 200115 VAL C, 300115 VAL C, 115 VAL C, 100115 VAL C, 100111 ALA C 2.18 13.95 3.72 0.91 0.1 98 10 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.29 14.86 2.28 0.24 0.03 99 11 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 1.73 18.07 1.8 0.02 0 100 12 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C 2.22 18.85 1.3 -0.14 0.33 101 13 100111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 2.16 20.44 -0.2 -0.61 1.33 105 14 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C 2.18 22.13 1.3 -0.14 0.33 101 15 100111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 2.43 23.27 -0.2 -0.61 1.33 105 16 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 1.44 32.69 -0.7 -0.77 1.66 107 17 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 300104 GLY C 4.18 33.28 -0.64 -0.78 2 107 18 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 300100 ILE C 4.55 33.72 0.34 -0.25 1.35 106 19 200107 THR C, 300100 ILE C, 100 ILE C, 100100 ILE C, 200100 ILE C 4.58 34.36 3.46 1.29 0.44 103 20 100107 THR C, 200107 THR C, 100 ILE C, 100100 ILE C, 200100 ILE C 4.66 34.54 2.42 0.78 0.74 104 21 107 THR C, 100107 THR C, 300107 THR C, 300100 ILE C, 100100 ILE C 4.64 34.74 1.38 0.26 1.05 105 22 107 THR C, 300107 THR C, 300104 GLY C, 300100 ILE C, 100100 ILE C 4.09 35.65 1.44 0.26 1.39 105 23 300107 THR C, 300104 GLY C, 300100 ILE C, 100100 ILE C 3.84 35.97 1.98 0.51 1.33 104 24 300104 GLY C, 300100 ILE C, 100100 ILE C, 300103 PHE C 3.58 36.33 2.85 1.04 1 85 25 300104 GLY C, 300100 ILE C, 300103 PHE C 3.15 37.4 2.3 0.79 1.29 77 26 107 THR C, 300104 GLY C, 300100 ILE C 1.65 40.78 1.13 0.08 1.72 105 27 107 THR C, 300104 GLY C, 300100 ILE C, 103 PHE C 1.45 41.57 0.75 -0.14 2.14 105 28 107 THR C, 300100 ILE C, 103 PHE C 1.46 42.7 1.13 0.08 1.72 105 29 107 THR C, 300100 ILE C, 103 PHE C, 300101 THR C 1.82 43.43 0.75 -0.14 2.14 105 30 107 THR C, 103 PHE C, 300101 THR C, 300100 ILE C 1.94 43.55 -0.48 -0.79 2.95 107 31 107 THR C, 103 PHE C, 300101 THR C, 300100 ILE C, 106 VAL C 1.96 43.69 0.46 -0.41 2.39 102 32 107 THR C, 103 PHE C, 300101 THR C, 106 VAL C 1.84 44.61 0.68 -0.31 2.14 102 33 107 THR C, 300101 THR C, 106 VAL C 1.83 44.86 1.03 -0.15 1.72 102 34 107 THR C, 300101 THR C, 106 VAL C, 110 LEU C 1.81 45.47 1.73 0.18 1.33 86 35 106 VAL C, 110 LEU C, 300101 THR C 1.21 49.89 2.43 0.5 0.64 86 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
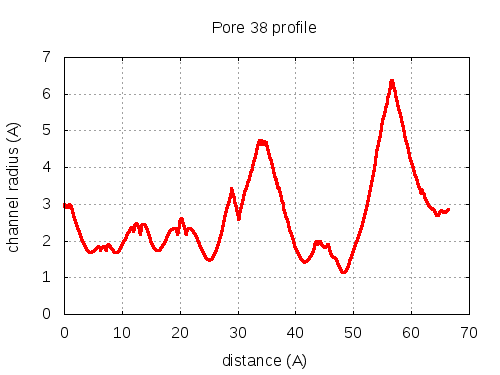
Unique lining residues set - all
119 GLN C, 100119 GLN C, 200115 VAL C, 300115 VAL C, 300119 GLN C, 115 VAL C, 100115 VAL C, 200119 GLN C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300108 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100104 GLY C, 100100 ILE C, 100 ILE C, 200100 ILE C, 300100 ILE C, 104 GLY C, 103 PHE C, 200103 PHE C, 101 THR C, 100 ILE C, 200106 VAL C, 200110 LEU C, 101 THR C
Unique lining residues set - sidechains
119 GLN C, 100119 GLN C, 200115 VAL C, 300115 VAL C, 300119 GLN C, 115 VAL C, 100115 VAL C, 200119 GLN C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100100 ILE C, 100 ILE C, 200100 ILE C, 300100 ILE C, 103 PHE C, 200106 VAL C, 200110 LEU C, 101 THR C
Physicochemical properties of lining side-chains
Charge: 0 (0-0)
Hydropathy: 1.5
Hydrophobicity: 0.14
Polarity: 1.08
Mutability: 94
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 119 GLN C, 100119 GLN C, 200115 VAL C, 300115 VAL C, 300119 GLN C 3.01 0.07 -0.42 -0.21 2.17 89 2 200115 VAL C, 300115 VAL C, 300119 GLN C, 115 VAL C, 100115 VAL C 2.91 0.46 2.66 0.68 0.81 95 3 119 GLN C, 100119 GLN C, 300119 GLN C, 200119 GLN C 2.9 1.71 -3.5 -1.1 3.53 84 4 119 GLN C, 200115 VAL C, 300115 VAL C, 115 VAL C, 100115 VAL C 2.2 3.56 2.66 0.68 0.81 95 5 200115 VAL C, 300115 VAL C, 115 VAL C, 100115 VAL C 1.71 10.89 4.2 1.13 0.13 98 6 200115 VAL C, 300115 VAL C, 115 VAL C, 100115 VAL C, 100111 ALA C 2.28 11.47 3.72 0.91 0.1 98 7 115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.27 11.96 2.28 0.24 0.03 99 8 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.29 12.41 2.28 0.24 0.03 99 9 200115 VAL C, 300115 VAL C, 115 VAL C, 100115 VAL C, 111 ALA C 2.41 12.93 3.72 0.91 0.1 98 10 200115 VAL C, 300115 VAL C, 115 VAL C, 100115 VAL C, 300111 ALA C 2.19 14.37 3.72 0.91 0.1 98 11 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.15 15.42 2.28 0.24 0.03 99 12 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 1.75 17.69 1.8 0.02 0 100 13 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300108 ALA C 2.07 18.6 1.36 -0.14 0.68 100 14 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C 2.39 19.19 1.3 -0.14 0.33 101 15 300111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 2.17 20.34 -0.2 -0.61 1.33 105 16 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300107 THR C 2.2 22.1 1.3 -0.14 0.33 101 17 100111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 2.27 23.31 -0.2 -0.61 1.33 105 18 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 1.44 32.38 -0.7 -0.77 1.66 107 19 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100104 GLY C 4.06 33.53 -0.64 -0.78 2 107 20 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100100 ILE C 4.74 33.8 0.34 -0.25 1.35 106 21 107 THR C, 100100 ILE C, 100 ILE C, 200100 ILE C, 300100 ILE C 4.62 34.26 3.46 1.29 0.44 103 22 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100100 ILE C 4.69 34.3 0.34 -0.25 1.35 106 23 100107 THR C, 200107 THR C, 300107 THR C, 100100 ILE C, 200100 ILE C 4.63 34.56 1.38 0.26 1.05 105 24 107 THR C, 200107 THR C, 300107 THR C, 100 ILE C, 300100 ILE C 4.65 34.75 1.38 0.26 1.05 105 25 107 THR C, 200107 THR C, 100 ILE C, 300100 ILE C, 104 GLY C 4.08 35.7 1.44 0.26 1.39 105 26 107 THR C, 100 ILE C, 300100 ILE C, 104 GLY C 3.82 36.02 1.98 0.51 1.33 104 27 100 ILE C, 300100 ILE C, 104 GLY C, 103 PHE C 3.57 36.4 2.85 1.04 1 85 28 100 ILE C, 104 GLY C, 103 PHE C 3.18 37.4 2.3 0.79 1.29 77 29 200107 THR C, 100 ILE C, 104 GLY C 1.67 40.84 1.13 0.08 1.72 105 30 200107 THR C, 100 ILE C, 104 GLY C, 200103 PHE C 1.45 41.65 0.75 -0.14 2.14 105 31 200107 THR C, 100 ILE C, 200103 PHE C 1.46 42.79 1.13 0.08 1.72 105 32 200107 THR C, 100 ILE C, 200103 PHE C, 101 THR C 1.84 43.53 0.75 -0.14 2.14 105 33 200107 THR C, 200103 PHE C, 101 THR C, 100 ILE C, 200106 VAL C 1.95 43.82 0.46 -0.41 2.39 102 34 200107 THR C, 200103 PHE C, 101 THR C, 200106 VAL C 1.87 44.54 0.68 -0.31 2.14 102 35 200107 THR C, 101 THR C, 200106 VAL C 1.82 44.82 1.03 -0.15 1.72 102 36 200107 THR C, 101 THR C, 200106 VAL C, 200110 LEU C 1.74 45.67 1.73 0.18 1.33 86 37 200106 VAL C, 200110 LEU C, 101 THR C 1.22 49.94 2.43 0.5 0.64 86 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
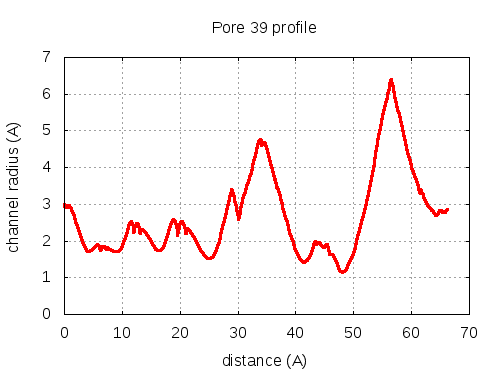
Unique lining residues set - all
119 GLN C, 100119 GLN C, 200115 VAL C, 300115 VAL C, 300119 GLN C, 115 VAL C, 100115 VAL C, 200119 GLN C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300108 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 104 GLY C, 100 ILE C, 100100 ILE C, 200100 ILE C, 300100 ILE C, 100104 GLY C, 100103 PHE C, 300103 PHE C, 100101 THR C, 100100 ILE C, 300106 VAL C, 300110 LEU C, 100101 THR C
Unique lining residues set - sidechains
119 GLN C, 100119 GLN C, 200115 VAL C, 300115 VAL C, 300119 GLN C, 115 VAL C, 100115 VAL C, 200119 GLN C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100 ILE C, 100100 ILE C, 200100 ILE C, 300100 ILE C, 100103 PHE C, 300106 VAL C, 300110 LEU C, 100101 THR C
Physicochemical properties of lining side-chains
Charge: 0 (0-0)
Hydropathy: 1.5
Hydrophobicity: 0.14
Polarity: 1.08
Mutability: 94
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 119 GLN C, 100119 GLN C, 200115 VAL C, 300115 VAL C, 300119 GLN C 3.01 0.07 -0.42 -0.21 2.17 89 2 200115 VAL C, 300115 VAL C, 300119 GLN C, 115 VAL C, 100115 VAL C 2.91 0.46 2.66 0.68 0.81 95 3 119 GLN C, 100119 GLN C, 300119 GLN C, 200119 GLN C 2.9 1.71 -3.5 -1.1 3.53 84 4 119 GLN C, 200115 VAL C, 300115 VAL C, 115 VAL C, 100115 VAL C 2.2 3.56 2.66 0.68 0.81 95 5 200115 VAL C, 300115 VAL C, 115 VAL C, 100115 VAL C 1.71 10.89 4.2 1.13 0.13 98 6 200115 VAL C, 300115 VAL C, 115 VAL C, 100115 VAL C, 100111 ALA C 2.28 11.47 3.72 0.91 0.1 98 7 115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.27 11.96 2.28 0.24 0.03 99 8 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.29 12.41 2.28 0.24 0.03 99 9 200115 VAL C, 300115 VAL C, 115 VAL C, 100115 VAL C, 111 ALA C 2.41 12.93 3.72 0.91 0.1 98 10 200115 VAL C, 300115 VAL C, 115 VAL C, 100115 VAL C, 300111 ALA C 2.19 14.37 3.72 0.91 0.1 98 11 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.15 15.42 2.28 0.24 0.03 99 12 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 1.75 17.69 1.8 0.02 0 100 13 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300108 ALA C 2.07 18.6 1.36 -0.14 0.68 100 14 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C 2.39 19.19 1.3 -0.14 0.33 101 15 300111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 2.17 20.34 -0.2 -0.61 1.33 105 16 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C 2.2 22.1 1.3 -0.14 0.33 101 17 100111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 2.27 23.31 -0.2 -0.61 1.33 105 18 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 1.44 32.38 -0.7 -0.77 1.66 107 19 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 104 GLY C 4.06 33.53 -0.64 -0.78 2 107 20 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100 ILE C 4.74 33.8 0.34 -0.25 1.35 106 21 100107 THR C, 100 ILE C, 100100 ILE C, 200100 ILE C, 300100 ILE C 4.62 34.26 3.46 1.29 0.44 103 22 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100 ILE C 4.69 34.3 0.34 -0.25 1.35 106 23 107 THR C, 200107 THR C, 300107 THR C, 100 ILE C, 300100 ILE C 4.63 34.56 1.38 0.26 1.05 105 24 100107 THR C, 200107 THR C, 300107 THR C, 100100 ILE C, 200100 ILE C 4.65 34.75 1.38 0.26 1.05 105 25 100107 THR C, 300107 THR C, 100100 ILE C, 200100 ILE C, 100104 GLY C 4.08 35.7 1.44 0.26 1.39 105 26 100107 THR C, 100100 ILE C, 200100 ILE C, 100104 GLY C 3.82 36.02 1.98 0.51 1.33 104 27 100100 ILE C, 200100 ILE C, 100104 GLY C, 100103 PHE C 3.57 36.4 2.85 1.04 1 85 28 100100 ILE C, 100104 GLY C, 100103 PHE C 3.18 37.4 2.3 0.79 1.29 77 29 300107 THR C, 100100 ILE C, 100104 GLY C 1.67 40.84 1.13 0.08 1.72 105 30 300107 THR C, 100100 ILE C, 100104 GLY C, 300103 PHE C 1.45 41.65 0.75 -0.14 2.14 105 31 300107 THR C, 100100 ILE C, 300103 PHE C 1.46 42.79 1.13 0.08 1.72 105 32 300107 THR C, 100100 ILE C, 300103 PHE C, 100101 THR C 1.84 43.53 0.75 -0.14 2.14 105 33 300107 THR C, 300103 PHE C, 100101 THR C, 100100 ILE C, 300106 VAL C 1.95 43.82 0.46 -0.41 2.39 102 34 300107 THR C, 300103 PHE C, 100101 THR C, 300106 VAL C 1.87 44.54 0.68 -0.31 2.14 102 35 300107 THR C, 100101 THR C, 300106 VAL C 1.82 44.82 1.03 -0.15 1.72 102 36 300107 THR C, 100101 THR C, 300106 VAL C, 300110 LEU C 1.74 45.67 1.73 0.18 1.33 86 37 300106 VAL C, 300110 LEU C, 100101 THR C 1.22 49.94 2.43 0.5 0.64 86 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
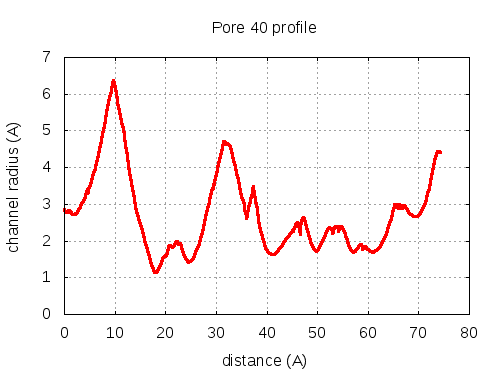
Unique lining residues set - all
119 GLN C, 100119 GLN C, 200115 VAL C, 300115 VAL C, 300119 GLN C, 115 VAL C, 100115 VAL C, 200119 GLN C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 200104 GLY C, 100100 ILE C, 100 ILE C, 200100 ILE C, 300100 ILE C, 200103 PHE C, 100103 PHE C, 200101 THR C, 100106 VAL C, 200100 ILE C, 100110 LEU C, 200101 THR C
Unique lining residues set - sidechains
119 GLN C, 100119 GLN C, 200115 VAL C, 300115 VAL C, 300119 GLN C, 115 VAL C, 100115 VAL C, 200119 GLN C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100100 ILE C, 100 ILE C, 200100 ILE C, 300100 ILE C, 200103 PHE C, 100106 VAL C, 100110 LEU C, 200101 THR C
Physicochemical properties of lining side-chains
Charge: 0 (0-0)
Hydropathy: 1.5
Hydrophobicity: 0.16
Polarity: 1.02
Mutability: 94
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 119 GLN C, 100119 GLN C, 200115 VAL C, 300115 VAL C, 300119 GLN C 3.01 0.08 -0.42 -0.21 2.17 89 2 200115 VAL C, 300115 VAL C, 300119 GLN C, 115 VAL C, 100115 VAL C 2.91 0.3 2.66 0.68 0.81 95 3 119 GLN C, 100119 GLN C, 200115 VAL C, 300115 VAL C, 300119 GLN C 2.98 0.49 -0.42 -0.21 2.17 89 4 119 GLN C, 100119 GLN C, 300119 GLN C, 200119 GLN C 2.91 1.25 -3.5 -1.1 3.53 84 5 119 GLN C, 200115 VAL C, 300115 VAL C, 300119 GLN C, 115 VAL C 2.89 1.96 1.12 0.24 1.49 92 6 119 GLN C, 200115 VAL C, 300115 VAL C, 115 VAL C, 100115 VAL C 2.57 2.91 2.66 0.68 0.81 95 7 200115 VAL C, 300115 VAL C, 115 VAL C, 100115 VAL C 1.73 5.64 4.2 1.13 0.13 98 8 200115 VAL C, 300115 VAL C, 115 VAL C 1.86 6.06 4.2 1.13 0.13 98 9 200115 VAL C, 300115 VAL C, 115 VAL C, 100115 VAL C 1.74 7.73 4.2 1.13 0.13 98 10 300115 VAL C, 115 VAL C, 100115 VAL C 1.81 8.36 4.2 1.13 0.13 98 11 200115 VAL C, 300115 VAL C, 115 VAL C, 100115 VAL C 1.7 10.83 4.2 1.13 0.13 98 12 200115 VAL C, 300115 VAL C, 115 VAL C, 100115 VAL C, 100111 ALA C 2.19 11.47 3.72 0.91 0.1 98 13 300115 VAL C, 115 VAL C, 100115 VAL C, 100111 ALA C, 111 ALA C 2.44 11.84 3.24 0.69 0.08 98 14 115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.25 12.03 2.28 0.24 0.03 99 15 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.28 12.48 2.28 0.24 0.03 99 16 300115 VAL C, 115 VAL C, 100111 ALA C, 111 ALA C, 300111 ALA C 2.52 12.78 2.76 0.46 0.05 99 17 200115 VAL C, 300115 VAL C, 115 VAL C, 100115 VAL C, 111 ALA C 2.39 13.01 3.72 0.91 0.1 98 18 200115 VAL C, 300115 VAL C, 115 VAL C, 100115 VAL C, 300111 ALA C 2.21 13.73 3.72 0.91 0.1 98 19 300115 VAL C, 115 VAL C, 100111 ALA C, 111 ALA C, 300111 ALA C 2.34 14.57 2.76 0.46 0.05 99 20 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.11 15.68 2.28 0.24 0.03 99 21 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 1.78 17.98 1.8 0.02 0 100 22 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C 2.15 19.36 1.3 -0.14 0.33 101 23 300111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 2.18 20.23 -0.2 -0.61 1.33 105 24 100111 ALA C, 300111 ALA C, 107 THR C, 100107 THR C, 300107 THR C 2.63 20.61 0.3 -0.45 1 104 25 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300107 THR C 2.19 21.21 1.3 -0.14 0.33 101 26 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 100107 THR C 2.39 21.81 1.3 -0.14 0.33 101 27 100111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 2.37 22.88 -0.2 -0.61 1.33 105 28 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 1.52 29.64 -0.7 -0.77 1.66 107 29 107 THR C, 100107 THR C, 300107 THR C 2.96 29.92 -0.7 -0.77 1.66 107 30 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 2.61 30.6 -0.7 -0.77 1.66 107 31 107 THR C, 100107 THR C, 300107 THR C 2.95 31.16 -0.7 -0.77 1.66 107 32 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 3.3 32.58 -0.7 -0.77 1.66 107 33 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 200104 GLY C 4.08 33.75 -0.64 -0.78 2 107 34 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100100 ILE C 4.69 34.01 0.34 -0.25 1.35 106 35 300107 THR C, 100100 ILE C, 100 ILE C, 200100 ILE C, 300100 ILE C 4.65 34.4 3.46 1.29 0.44 103 36 107 THR C, 100107 THR C, 200107 THR C, 100100 ILE C, 100 ILE C 4.7 34.47 1.38 0.26 1.05 105 37 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100 ILE C 4.71 34.5 0.34 -0.25 1.35 106 38 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 200100 ILE C 4.69 34.6 0.34 -0.25 1.35 106 39 107 THR C, 100107 THR C, 200107 THR C, 100100 ILE C, 100 ILE C 4.7 34.64 1.38 0.26 1.05 105 40 107 THR C, 300107 THR C, 100100 ILE C, 100 ILE C, 300100 ILE C 4.62 34.82 2.42 0.78 0.74 104 41 107 THR C, 100107 THR C, 200107 THR C, 100 ILE C, 200100 ILE C 4.6 35.21 1.38 0.26 1.05 105 42 100107 THR C, 200107 THR C, 200104 GLY C, 100 ILE C, 200100 ILE C 4.02 36.16 1.44 0.26 1.39 105 43 200107 THR C, 200104 GLY C, 100 ILE C, 200100 ILE C 3.88 36.32 1.98 0.51 1.33 104 44 200104 GLY C, 100 ILE C, 200100 ILE C, 200103 PHE C 3.55 36.77 2.85 1.04 1 85 45 200104 GLY C, 200100 ILE C, 200103 PHE C 3.17 37.8 2.3 0.79 1.29 77 46 100107 THR C, 200104 GLY C, 200100 ILE C 1.6 41.41 1.13 0.08 1.72 105 47 100107 THR C, 200104 GLY C, 200100 ILE C, 100103 PHE C 1.43 42.22 0.75 -0.14 2.14 105 48 100107 THR C, 200100 ILE C, 100103 PHE C 1.5 43.26 1.13 0.08 1.72 105 49 100107 THR C, 200100 ILE C, 100103 PHE C, 200101 THR C 1.89 43.96 0.75 -0.14 2.14 105 50 100107 THR C, 100103 PHE C, 200101 THR C, 100106 VAL C, 200100 ILE C 1.96 44.11 0.46 -0.41 2.39 102 51 100107 THR C, 100103 PHE C, 200101 THR C, 100106 VAL C 1.83 45.09 0.68 -0.31 2.14 102 52 100107 THR C, 200101 THR C, 100106 VAL C 1.84 45.35 1.03 -0.15 1.72 102 53 100107 THR C, 200101 THR C, 100106 VAL C, 100110 LEU C 1.76 45.98 1.73 0.18 1.33 86 54 100106 VAL C, 100110 LEU C, 200101 THR C 1.22 50.3 2.43 0.5 0.64 86 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
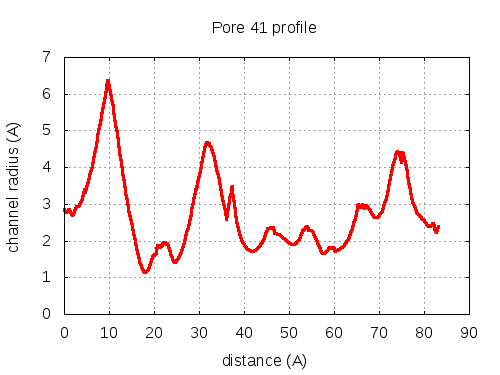
Unique lining residues set - all
106 VAL C, 107 THR C, 110 LEU C, 300101 THR C, 103 PHE C, 300100 ILE C, 300100 ILE C, 300104 GLY C, 300103 PHE C, 100100 ILE C, 300107 THR C, 100107 THR C, 100 ILE C, 200100 ILE C, 200107 THR C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C, 100124 HIS C, 124 HIS C, 200124 HIS C, 300124 HIS C, 100124 HIS C, 300122 ARG C
Unique lining residues set - sidechains
106 VAL C, 107 THR C, 110 LEU C, 300100 ILE C, 300103 PHE C, 100100 ILE C, 300107 THR C, 100107 THR C, 100 ILE C, 200100 ILE C, 200107 THR C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C, 100124 HIS C, 124 HIS C, 200124 HIS C, 300124 HIS C, 300122 ARG C
Physicochemical properties of lining side-chains
Charge: 1 (1-0)
Hydropathy: 0.3
Hydrophobicity: -0.04
Polarity: 6.27
Mutability: 93
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 106 VAL C, 107 THR C, 110 LEU C, 300101 THR C 1.86 0.59 1.73 0.18 1.33 86 2 106 VAL C, 107 THR C, 300101 THR C 1.83 0.85 1.03 -0.15 1.72 102 3 106 VAL C, 107 THR C, 300101 THR C, 103 PHE C 1.84 1.57 0.68 -0.31 2.14 102 4 106 VAL C, 107 THR C, 300101 THR C, 103 PHE C, 300100 ILE C 1.96 1.7 0.46 -0.41 2.39 102 5 107 THR C, 300101 THR C, 103 PHE C, 300100 ILE C 1.94 1.8 -0.48 -0.79 2.95 107 6 107 THR C, 300101 THR C, 103 PHE C, 300100 ILE C 1.87 2.77 0.75 -0.14 2.14 105 7 107 THR C, 103 PHE C, 300100 ILE C 1.47 4.29 1.13 0.08 1.72 105 8 107 THR C, 103 PHE C, 300100 ILE C, 300104 GLY C 1.45 5.19 0.75 -0.14 2.14 105 9 107 THR C, 300100 ILE C, 300104 GLY C 1.67 8.13 1.13 0.08 1.72 105 10 300100 ILE C, 300104 GLY C, 300103 PHE C 3.21 8.81 2.3 0.79 1.29 77 11 300100 ILE C, 300104 GLY C, 300103 PHE C, 100100 ILE C 3.56 9.28 2.85 1.04 1 85 12 300100 ILE C, 300104 GLY C, 100100 ILE C, 300107 THR C 3.84 9.69 1.98 0.51 1.33 104 13 107 THR C, 300100 ILE C, 300104 GLY C, 100100 ILE C, 300107 THR C 4.09 10.44 1.44 0.26 1.39 105 14 107 THR C, 300100 ILE C, 100100 ILE C, 300107 THR C, 100107 THR C 4.55 10.83 1.38 0.26 1.05 105 15 300100 ILE C, 100100 ILE C, 100 ILE C, 200100 ILE C, 200107 THR C 4.58 11.73 3.46 1.29 0.44 103 16 107 THR C, 300100 ILE C, 300107 THR C, 100107 THR C, 200107 THR C 4.57 12.36 0.34 -0.25 1.35 106 17 107 THR C, 300104 GLY C, 300107 THR C, 100107 THR C, 200107 THR C 4.27 13.08 -0.64 -0.78 2 107 18 107 THR C, 300107 THR C, 100107 THR C, 200107 THR C 1.44 23.19 -0.7 -0.77 1.66 107 19 107 THR C, 300107 THR C, 100107 THR C, 200107 THR C, 100111 ALA C 2.49 23.88 -0.2 -0.61 1.33 105 20 107 THR C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.18 24.87 1.3 -0.14 0.33 101 21 300107 THR C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.54 25.24 1.3 -0.14 0.33 101 22 107 THR C, 300107 THR C, 100107 THR C, 200107 THR C, 100111 ALA C 2.21 27.11 -0.2 -0.61 1.33 105 23 107 THR C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.18 28.22 1.3 -0.14 0.33 101 24 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 1.76 30.55 1.8 0.02 0 100 25 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300115 VAL C 2.15 31.9 2.28 0.24 0.03 99 26 100111 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 2.18 32.69 3.72 0.91 0.1 98 27 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300115 VAL C 2.43 33.14 2.28 0.24 0.03 99 28 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 115 VAL C 2.28 33.73 2.28 0.24 0.03 99 29 100111 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 2.18 35.22 3.72 0.91 0.1 98 30 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 1.7 42.51 4.2 1.13 0.13 98 31 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 119 GLN C 2.19 44.04 2.66 0.68 0.81 95 32 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C 2.9 44.86 -3.5 -1.1 3.53 84 33 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 300119 GLN C 2.93 44.97 2.66 0.68 0.81 95 34 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 119 GLN C 2.9 45.16 2.66 0.68 0.81 95 35 200115 VAL C, 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C 2.99 45.25 -1.96 -0.65 2.85 86 36 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C 2.65 51.14 -3.5 -1.1 3.53 84 37 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C, 100124 HIS C 3.53 52.85 -3.44 -0.83 13.14 85 38 200119 GLN C, 100124 HIS C, 124 HIS C, 200124 HIS C, 300124 HIS C 4.4 53.18 -3.26 -0.01 41.99 89 39 119 GLN C, 100119 GLN C, 300119 GLN C, 100124 HIS C, 300124 HIS C 4.33 53.51 -3.38 -0.56 22.76 86 40 119 GLN C, 100119 GLN C, 200119 GLN C, 124 HIS C, 200124 HIS C 4.12 54.67 -3.38 -0.56 22.76 86 41 119 GLN C, 100119 GLN C, 300119 GLN C, 100124 HIS C, 300124 HIS C 4.11 55.49 -3.38 -0.56 22.76 86 42 100119 GLN C, 300119 GLN C, 100124 HIS C, 300124 HIS C 2.99 57.42 -3.35 -0.42 27.57 87 43 300119 GLN C, 100124 HIS C, 300124 HIS C 2.41 60.56 -3.3 -0.19 35.58 88 44 300119 GLN C, 300124 HIS C, 100124 HIS C, 300122 ARG C 2.25 61.49 -2.9 -0.52 27.63 86 45 300124 HIS C, 100124 HIS C, 300122 ARG C 2.25 62.08 -2.7 -0.32 35.66 87 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
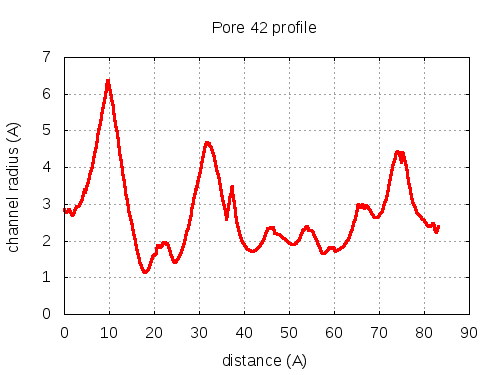
Unique lining residues set - all
106 VAL C, 107 THR C, 110 LEU C, 300101 THR C, 103 PHE C, 300100 ILE C, 300100 ILE C, 300104 GLY C, 300103 PHE C, 100100 ILE C, 300107 THR C, 100107 THR C, 100 ILE C, 200100 ILE C, 200107 THR C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C, 300124 HIS C, 124 HIS C, 100124 HIS C, 200124 HIS C, 100122 ARG C, 200124 HIS C
Unique lining residues set - sidechains
106 VAL C, 107 THR C, 110 LEU C, 300100 ILE C, 300103 PHE C, 100100 ILE C, 300107 THR C, 100107 THR C, 100 ILE C, 200100 ILE C, 200107 THR C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C, 300124 HIS C, 124 HIS C, 100124 HIS C, 200124 HIS C, 100122 ARG C,
Physicochemical properties of lining side-chains
Charge: 1 (1-0)
Hydropathy: 0
Hydrophobicity: -0.09
Polarity: 7.09
Mutability: 93
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 106 VAL C, 107 THR C, 110 LEU C, 300101 THR C 1.86 0.6 1.73 0.18 1.33 86 2 106 VAL C, 107 THR C, 300101 THR C 1.83 0.87 1.03 -0.15 1.72 102 3 106 VAL C, 107 THR C, 300101 THR C, 103 PHE C 1.84 1.59 0.68 -0.31 2.14 102 4 106 VAL C, 107 THR C, 300101 THR C, 103 PHE C, 300100 ILE C 1.96 1.72 0.46 -0.41 2.39 102 5 107 THR C, 300101 THR C, 103 PHE C, 300100 ILE C 1.91 2.43 0.75 -0.14 2.14 105 6 107 THR C, 103 PHE C, 300100 ILE C 1.44 4.61 1.13 0.08 1.72 105 7 107 THR C, 103 PHE C, 300100 ILE C, 300104 GLY C 1.5 5.54 0.75 -0.14 2.14 105 8 107 THR C, 300100 ILE C, 300104 GLY C 1.81 8.24 1.13 0.08 1.72 105 9 300100 ILE C, 300104 GLY C, 300103 PHE C 3.29 8.79 2.3 0.79 1.29 77 10 300100 ILE C, 300104 GLY C, 300103 PHE C, 100100 ILE C 3.56 9.26 2.85 1.04 1 85 11 300100 ILE C, 300104 GLY C, 100100 ILE C, 300107 THR C 3.83 9.67 1.98 0.51 1.33 104 12 107 THR C, 300100 ILE C, 300104 GLY C, 100100 ILE C, 300107 THR C 4.08 10.43 1.44 0.26 1.39 105 13 107 THR C, 300100 ILE C, 100100 ILE C, 300107 THR C, 100107 THR C 4.56 10.83 1.38 0.26 1.05 105 14 300100 ILE C, 100100 ILE C, 100 ILE C, 200100 ILE C, 200107 THR C 4.57 11.82 3.46 1.29 0.44 103 15 107 THR C, 300100 ILE C, 300107 THR C, 100107 THR C, 200107 THR C 4.6 12.48 0.34 -0.25 1.35 106 16 107 THR C, 300104 GLY C, 300107 THR C, 100107 THR C, 200107 THR C 4.18 13.22 -0.64 -0.78 2 107 17 107 THR C, 300107 THR C, 100107 THR C, 200107 THR C 1.5 22.72 -0.7 -0.77 1.66 107 18 107 THR C, 300107 THR C, 100107 THR C, 200107 THR C, 100111 ALA C 2.31 23.65 -0.2 -0.61 1.33 105 19 107 THR C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.16 25.12 1.3 -0.14 0.33 101 20 107 THR C, 300107 THR C, 100107 THR C, 200107 THR C, 100111 ALA C 2.19 26.87 -0.2 -0.61 1.33 105 21 107 THR C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.18 27.97 1.3 -0.14 0.33 101 22 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 1.77 30.36 1.8 0.02 0 100 23 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300115 VAL C 2 31.85 2.28 0.24 0.03 99 24 100111 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 2.22 32.65 3.72 0.91 0.1 98 25 100111 ALA C, 111 ALA C, 300111 ALA C, 300115 VAL C, 115 VAL C 2.44 32.93 2.76 0.46 0.05 99 26 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300115 VAL C 2.39 33.12 2.28 0.24 0.03 99 27 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 115 VAL C 2.28 33.74 2.28 0.24 0.03 99 28 100111 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 2.12 35.29 3.72 0.91 0.1 98 29 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 1.7 42.91 4.2 1.13 0.13 98 30 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 119 GLN C 2.43 44.18 2.66 0.68 0.81 95 31 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C 2.92 44.79 -3.5 -1.1 3.53 84 32 300115 VAL C, 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C 3 44.91 -1.96 -0.65 2.85 86 33 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 300119 GLN C 2.9 45.12 2.66 0.68 0.81 95 34 200115 VAL C, 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C 2.99 45.21 -1.96 -0.65 2.85 86 35 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C 2.65 51.23 -3.5 -1.1 3.53 84 36 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C, 300124 HIS C 3.59 52.68 -3.44 -0.83 13.14 85 37 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C, 124 HIS C 4.4 52.86 -3.44 -0.83 13.14 85 38 100119 GLN C, 300124 HIS C, 124 HIS C, 100124 HIS C, 200124 HIS C 4.42 53.11 -3.26 -0.01 41.99 89 39 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C, 300124 HIS C 4.4 53.17 -3.44 -0.83 13.14 85 40 119 GLN C, 200119 GLN C, 300119 GLN C, 300124 HIS C, 124 HIS C 4.38 53.24 -3.38 -0.56 22.76 86 41 100119 GLN C, 200119 GLN C, 300119 GLN C, 100124 HIS C, 200124 HIS C 4.34 53.49 -3.38 -0.56 22.76 86 42 119 GLN C, 200119 GLN C, 300119 GLN C, 300124 HIS C, 124 HIS C 4.15 54.5 -3.38 -0.56 22.76 86 43 100119 GLN C, 200119 GLN C, 300119 GLN C, 100124 HIS C, 200124 HIS C 4.12 55.27 -3.38 -0.56 22.76 86 44 100119 GLN C, 200119 GLN C, 100124 HIS C, 200124 HIS C 3.13 57.22 -3.35 -0.42 27.57 87 45 100119 GLN C, 100124 HIS C, 200124 HIS C 2.4 60.83 -3.3 -0.19 35.58 88 46 100119 GLN C, 100124 HIS C, 100122 ARG C, 200124 HIS C 2.25 61.51 -2.9 -0.52 27.63 86 47 100124 HIS C, 100122 ARG C, 200124 HIS C 2.25 62.11 -2.7 -0.32 35.66 87 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
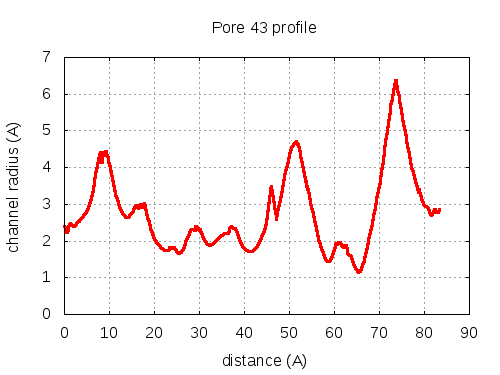
Unique lining residues set - all
100101 THR C, 300106 VAL C, 300107 THR C, 300110 LEU C, 300103 PHE C, 100100 ILE C, 100100 ILE C, 100104 GLY C, 100103 PHE C, 200100 ILE C, 100107 THR C, 200107 THR C, 100 ILE C, 107 THR C, 300100 ILE C, 104 GLY C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C, 100124 HIS C, 124 HIS C, 200124 HIS C, 300124 HIS C, 100124 HIS C, 300122 ARG C
Unique lining residues set - sidechains
300106 VAL C, 300107 THR C, 300110 LEU C, 100100 ILE C, 100103 PHE C, 200100 ILE C, 100107 THR C, 200107 THR C, 100 ILE C, 107 THR C, 300100 ILE C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C, 100124 HIS C, 124 HIS C, 200124 HIS C, 300124 HIS C, 300122 ARG C
Physicochemical properties of lining side-chains
Charge: 1 (1-0)
Hydropathy: 0.5
Hydrophobicity: 0.02
Polarity: 6.05
Mutability: 93
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 100101 THR C, 300106 VAL C, 300107 THR C, 300110 LEU C 1.86 0.6 1.73 0.18 1.33 86 2 100101 THR C, 300106 VAL C, 300107 THR C 1.83 0.87 1.03 -0.15 1.72 102 3 100101 THR C, 300106 VAL C, 300107 THR C, 300103 PHE C 1.84 1.59 0.68 -0.31 2.14 102 4 100101 THR C, 300106 VAL C, 300107 THR C, 300103 PHE C, 100100 ILE C 1.96 1.72 0.46 -0.41 2.39 102 5 100101 THR C, 300107 THR C, 300103 PHE C, 100100 ILE C 1.91 2.43 0.75 -0.14 2.14 105 6 300107 THR C, 300103 PHE C, 100100 ILE C 1.44 4.61 1.13 0.08 1.72 105 7 300107 THR C, 300103 PHE C, 100100 ILE C, 100104 GLY C 1.5 5.54 0.75 -0.14 2.14 105 8 300107 THR C, 100100 ILE C, 100104 GLY C 1.81 8.24 1.13 0.08 1.72 105 9 100100 ILE C, 100104 GLY C, 100103 PHE C 3.29 8.8 2.3 0.79 1.29 77 10 100100 ILE C, 100104 GLY C, 100103 PHE C, 200100 ILE C 3.56 9.27 2.85 1.04 1 85 11 100100 ILE C, 100104 GLY C, 200100 ILE C, 100107 THR C 3.81 9.68 1.98 0.51 1.33 104 12 300107 THR C, 100100 ILE C, 100104 GLY C, 200100 ILE C, 100107 THR C 4.08 10.46 1.44 0.26 1.39 105 13 300107 THR C, 100100 ILE C, 200100 ILE C, 100107 THR C, 200107 THR C 4.54 10.77 1.38 0.26 1.05 105 14 300107 THR C, 200107 THR C, 100 ILE C, 107 THR C, 300100 ILE C 4.64 10.95 1.38 0.26 1.05 105 15 300107 THR C, 100107 THR C, 200107 THR C, 100 ILE C, 107 THR C 4.69 11.07 0.34 -0.25 1.35 106 16 100100 ILE C, 200100 ILE C, 100107 THR C, 100 ILE C, 300100 ILE C 4.64 12.06 3.46 1.29 0.44 103 17 300107 THR C, 100107 THR C, 200107 THR C, 107 THR C, 104 GLY C 4.13 13.49 -0.64 -0.78 2 107 18 300107 THR C, 100107 THR C, 200107 THR C, 107 THR C 1.55 23.05 -0.7 -0.77 1.66 107 19 300107 THR C, 100107 THR C, 200107 THR C, 107 THR C, 100111 ALA C 2.1 23.91 -0.2 -0.61 1.33 105 20 107 THR C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.21 25.29 1.3 -0.14 0.33 101 21 300107 THR C, 100107 THR C, 200107 THR C, 107 THR C, 300111 ALA C 2.16 27.15 -0.2 -0.61 1.33 105 22 107 THR C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.3 28.28 1.3 -0.14 0.33 101 23 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 1.79 30.66 1.8 0.02 0 100 24 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300115 VAL C 2.1 31.54 2.28 0.24 0.03 99 25 100111 ALA C, 111 ALA C, 300111 ALA C, 300115 VAL C, 115 VAL C 2.28 32.02 2.76 0.46 0.05 99 26 300111 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 2.22 32.53 3.72 0.91 0.1 98 27 111 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 2.44 32.83 3.72 0.91 0.1 98 28 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300115 VAL C 2.27 33.28 2.28 0.24 0.03 99 29 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 115 VAL C 2.27 33.95 2.28 0.24 0.03 99 30 100111 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 2.12 35.54 3.72 0.91 0.1 98 31 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 1.71 43.17 4.2 1.13 0.13 98 32 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 119 GLN C 2.54 44.33 2.66 0.68 0.81 95 33 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C 2.89 44.92 -3.5 -1.1 3.53 84 34 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 300119 GLN C 2.94 45.06 2.66 0.68 0.81 95 35 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 119 GLN C 2.9 45.24 2.66 0.68 0.81 95 36 200115 VAL C, 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C 2.99 45.34 -1.96 -0.65 2.85 86 37 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C 2.66 51.46 -3.5 -1.1 3.53 84 38 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C, 100124 HIS C 3.64 52.85 -3.44 -0.83 13.14 85 39 200119 GLN C, 100124 HIS C, 124 HIS C, 200124 HIS C, 300124 HIS C 4.42 53.26 -3.26 -0.01 41.99 89 40 119 GLN C, 100119 GLN C, 300119 GLN C, 100124 HIS C, 300124 HIS C 4.33 53.55 -3.38 -0.56 22.76 86 41 119 GLN C, 100119 GLN C, 200119 GLN C, 124 HIS C, 200124 HIS C 4.15 54.57 -3.38 -0.56 22.76 86 42 119 GLN C, 100119 GLN C, 300119 GLN C, 100124 HIS C, 300124 HIS C 4.12 55.34 -3.38 -0.56 22.76 86 43 100119 GLN C, 300119 GLN C, 100124 HIS C, 300124 HIS C 3.13 57.29 -3.35 -0.42 27.57 87 44 300119 GLN C, 100124 HIS C, 300124 HIS C 2.4 60.9 -3.3 -0.19 35.58 88 45 300119 GLN C, 300124 HIS C, 100124 HIS C, 300122 ARG C 2.25 61.58 -2.9 -0.52 27.63 86 46 300124 HIS C, 100124 HIS C, 300122 ARG C 2.25 62.17 -2.7 -0.32 35.66 87 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
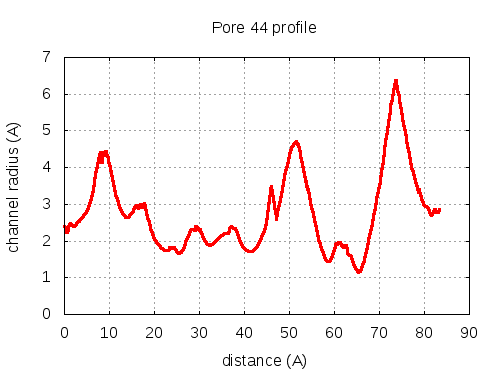
Unique lining residues set - all
100101 THR C, 300106 VAL C, 300107 THR C, 300110 LEU C, 300103 PHE C, 100100 ILE C, 100100 ILE C, 100104 GLY C, 100103 PHE C, 200100 ILE C, 100107 THR C, 200107 THR C, 100 ILE C, 107 THR C, 300100 ILE C, 104 GLY C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300108 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C, 300124 HIS C, 124 HIS C, 100124 HIS C, 200124 HIS C, 100122 ARG C, 200124 HIS C
Unique lining residues set - sidechains
300106 VAL C, 300107 THR C, 300110 LEU C, 100100 ILE C, 100103 PHE C, 200100 ILE C, 100107 THR C, 200107 THR C, 100 ILE C, 107 THR C, 300100 ILE C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C, 300124 HIS C, 124 HIS C, 100124 HIS C, 200124 HIS C, 100122 ARG C,
Physicochemical properties of lining side-chains
Charge: 1 (1-0)
Hydropathy: 0.1
Hydrophobicity: -0.06
Polarity: 7.43
Mutability: 93
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 100101 THR C, 300106 VAL C, 300107 THR C, 300110 LEU C 1.86 0.61 1.73 0.18 1.33 86 2 100101 THR C, 300106 VAL C, 300107 THR C 1.83 0.88 1.03 -0.15 1.72 102 3 100101 THR C, 300106 VAL C, 300107 THR C, 300103 PHE C 1.85 1.47 0.68 -0.31 2.14 102 4 100101 THR C, 300106 VAL C, 300107 THR C, 300103 PHE C, 100100 ILE C 1.96 1.74 0.46 -0.41 2.39 102 5 100101 THR C, 300107 THR C, 300103 PHE C, 100100 ILE C 1.91 2.6 0.75 -0.14 2.14 105 6 300107 THR C, 300103 PHE C, 100100 ILE C 1.44 5.03 1.13 0.08 1.72 105 7 300107 THR C, 100100 ILE C, 100104 GLY C 1.61 8.14 1.13 0.08 1.72 105 8 100100 ILE C, 100104 GLY C, 100103 PHE C 3.22 8.8 2.3 0.79 1.29 77 9 100100 ILE C, 100104 GLY C, 100103 PHE C, 200100 ILE C 3.56 9.29 2.85 1.04 1 85 10 100100 ILE C, 100104 GLY C, 200100 ILE C, 100107 THR C 3.81 9.72 1.98 0.51 1.33 104 11 300107 THR C, 100100 ILE C, 100104 GLY C, 200100 ILE C, 100107 THR C 4.1 10.51 1.44 0.26 1.39 105 12 300107 THR C, 100100 ILE C, 200100 ILE C, 100107 THR C, 200107 THR C 4.58 10.79 1.38 0.26 1.05 105 13 300107 THR C, 200107 THR C, 100 ILE C, 107 THR C, 300100 ILE C 4.64 10.91 1.38 0.26 1.05 105 14 300107 THR C, 100107 THR C, 200107 THR C, 100 ILE C, 107 THR C 4.68 10.99 0.34 -0.25 1.35 106 15 100100 ILE C, 200100 ILE C, 100107 THR C, 100 ILE C, 300100 ILE C 4.64 11.8 3.46 1.29 0.44 103 16 300107 THR C, 100107 THR C, 200107 THR C, 100 ILE C, 107 THR C 4.61 12.41 0.34 -0.25 1.35 106 17 300107 THR C, 100107 THR C, 200107 THR C, 107 THR C, 104 GLY C 4.34 13.16 -0.64 -0.78 2 107 18 300107 THR C, 100107 THR C, 200107 THR C, 107 THR C 1.56 23.02 -0.7 -0.77 1.66 107 19 300107 THR C, 100107 THR C, 200107 THR C, 107 THR C, 100111 ALA C 2.08 23.9 -0.2 -0.61 1.33 105 20 107 THR C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.22 25.34 1.3 -0.14 0.33 101 21 300107 THR C, 100107 THR C, 200107 THR C, 107 THR C, 300111 ALA C 2.18 26.47 -0.2 -0.61 1.33 105 22 107 THR C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.41 27.43 1.3 -0.14 0.33 101 23 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300108 ALA C 2.17 28.64 1.36 -0.14 0.68 100 24 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 1.76 31 1.8 0.02 0 100 25 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300115 VAL C 2.29 31.76 2.28 0.24 0.03 99 26 300111 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 2.2 32.33 3.72 0.91 0.1 98 27 111 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 2.31 32.93 3.72 0.91 0.1 98 28 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300115 VAL C 2.33 33.25 2.28 0.24 0.03 99 29 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 115 VAL C 2.28 34.38 2.28 0.24 0.03 99 30 100111 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 2.16 35.24 3.72 0.91 0.1 98 31 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 1.7 43.25 4.2 1.13 0.13 98 32 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 119 GLN C 2.55 44 2.66 0.68 0.81 95 33 200115 VAL C, 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C 2.84 44.37 -1.96 -0.65 2.85 86 34 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C 2.92 44.95 -3.5 -1.1 3.53 84 35 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 300119 GLN C 2.9 45.27 2.66 0.68 0.81 95 36 200115 VAL C, 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C 2.99 45.37 -1.96 -0.65 2.85 86 37 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C 2.67 51.39 -3.5 -1.1 3.53 84 38 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C, 300124 HIS C 3.61 52.84 -3.44 -0.83 13.14 85 39 200119 GLN C, 300124 HIS C, 124 HIS C, 100124 HIS C, 200124 HIS C 4.44 53 -3.26 -0.01 41.99 89 40 100119 GLN C, 300124 HIS C, 124 HIS C, 100124 HIS C, 200124 HIS C 4.42 53.25 -3.26 -0.01 41.99 89 41 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C, 300124 HIS C 4.39 53.29 -3.44 -0.83 13.14 85 42 119 GLN C, 200119 GLN C, 300119 GLN C, 300124 HIS C, 124 HIS C 4.38 53.37 -3.38 -0.56 22.76 86 43 100119 GLN C, 200119 GLN C, 300119 GLN C, 100124 HIS C, 200124 HIS C 4.33 53.69 -3.38 -0.56 22.76 86 44 119 GLN C, 200119 GLN C, 300119 GLN C, 300124 HIS C, 124 HIS C 4.18 54.42 -3.38 -0.56 22.76 86 45 100119 GLN C, 200119 GLN C, 300119 GLN C, 100124 HIS C, 200124 HIS C 3.86 56.05 -3.38 -0.56 22.76 86 46 100119 GLN C, 200119 GLN C, 100124 HIS C, 200124 HIS C 2.83 58.08 -3.35 -0.42 27.57 87 47 100119 GLN C, 100124 HIS C, 200124 HIS C 2.39 60.94 -3.3 -0.19 35.58 88 48 100119 GLN C, 100124 HIS C, 100122 ARG C, 200124 HIS C 2.26 61.64 -2.9 -0.52 27.63 86 49 100124 HIS C, 100122 ARG C, 200124 HIS C 2.24 62.25 -2.7 -0.32 35.66 87 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
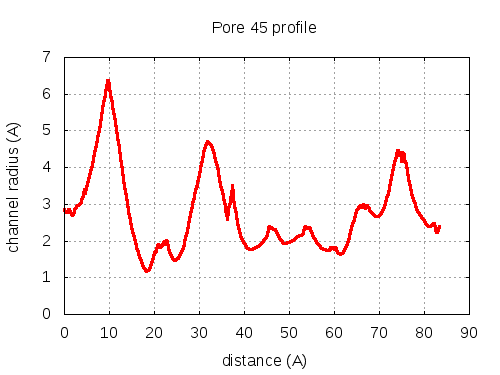
Unique lining residues set - all
101 THR C, 200106 VAL C, 200107 THR C, 200110 LEU C, 200103 PHE C, 100 ILE C, 100 ILE C, 104 GLY C, 103 PHE C, 300100 ILE C, 107 THR C, 300107 THR C, 100100 ILE C, 100107 THR C, 200100 ILE C, 100104 GLY C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300108 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C, 300124 HIS C, 124 HIS C, 100124 HIS C, 200124 HIS C, 100122 ARG C, 200124 HIS C
Unique lining residues set - sidechains
200106 VAL C, 200107 THR C, 200110 LEU C, 100 ILE C, 103 PHE C, 300100 ILE C, 107 THR C, 300107 THR C, 100100 ILE C, 100107 THR C, 200100 ILE C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C, 300124 HIS C, 124 HIS C, 100124 HIS C, 200124 HIS C, 100122 ARG C,
Physicochemical properties of lining side-chains
Charge: 1 (1-0)
Hydropathy: 0.1
Hydrophobicity: -0.06
Polarity: 7.43
Mutability: 93
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 101 THR C, 200106 VAL C, 200107 THR C, 200110 LEU C 1.86 0.61 1.73 0.18 1.33 86 2 101 THR C, 200106 VAL C, 200107 THR C 1.83 0.88 1.03 -0.15 1.72 102 3 101 THR C, 200106 VAL C, 200107 THR C, 200103 PHE C 1.85 1.47 0.68 -0.31 2.14 102 4 101 THR C, 200106 VAL C, 200107 THR C, 200103 PHE C, 100 ILE C 1.96 1.74 0.46 -0.41 2.39 102 5 101 THR C, 200107 THR C, 200103 PHE C, 100 ILE C 1.91 2.6 0.75 -0.14 2.14 105 6 200107 THR C, 200103 PHE C, 100 ILE C 1.44 5.03 1.13 0.08 1.72 105 7 200107 THR C, 100 ILE C, 104 GLY C 1.61 8.14 1.13 0.08 1.72 105 8 100 ILE C, 104 GLY C, 103 PHE C 3.22 8.8 2.3 0.79 1.29 77 9 100 ILE C, 104 GLY C, 103 PHE C, 300100 ILE C 3.56 9.29 2.85 1.04 1 85 10 100 ILE C, 104 GLY C, 300100 ILE C, 107 THR C 3.81 9.72 1.98 0.51 1.33 104 11 200107 THR C, 100 ILE C, 104 GLY C, 300100 ILE C, 107 THR C 4.1 10.51 1.44 0.26 1.39 105 12 200107 THR C, 100 ILE C, 300100 ILE C, 107 THR C, 300107 THR C 4.58 10.79 1.38 0.26 1.05 105 13 200107 THR C, 300107 THR C, 100100 ILE C, 100107 THR C, 200100 ILE C 4.64 10.91 1.38 0.26 1.05 105 14 200107 THR C, 107 THR C, 300107 THR C, 100100 ILE C, 100107 THR C 4.68 10.99 0.34 -0.25 1.35 106 15 100 ILE C, 300100 ILE C, 107 THR C, 100100 ILE C, 200100 ILE C 4.64 11.8 3.46 1.29 0.44 103 16 200107 THR C, 107 THR C, 300107 THR C, 100100 ILE C, 100107 THR C 4.61 12.41 0.34 -0.25 1.35 106 17 200107 THR C, 107 THR C, 300107 THR C, 100107 THR C, 100104 GLY C 4.34 13.16 -0.64 -0.78 2 107 18 200107 THR C, 107 THR C, 300107 THR C, 100107 THR C 1.56 23.02 -0.7 -0.77 1.66 107 19 200107 THR C, 107 THR C, 300107 THR C, 100107 THR C, 100111 ALA C 2.08 23.9 -0.2 -0.61 1.33 105 20 300107 THR C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.22 25.34 1.3 -0.14 0.33 101 21 200107 THR C, 107 THR C, 300107 THR C, 100107 THR C, 300111 ALA C 2.18 26.47 -0.2 -0.61 1.33 105 22 107 THR C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.41 27.43 1.3 -0.14 0.33 101 23 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300108 ALA C 2.17 28.64 1.36 -0.14 0.68 100 24 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 1.76 31 1.8 0.02 0 100 25 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300115 VAL C 2.29 31.76 2.28 0.24 0.03 99 26 300111 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 2.2 32.33 3.72 0.91 0.1 98 27 111 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 2.31 32.93 3.72 0.91 0.1 98 28 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300115 VAL C 2.33 33.25 2.28 0.24 0.03 99 29 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 115 VAL C 2.28 34.38 2.28 0.24 0.03 99 30 100111 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 2.16 35.24 3.72 0.91 0.1 98 31 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 1.7 43.25 4.2 1.13 0.13 98 32 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 119 GLN C 2.55 44 2.66 0.68 0.81 95 33 200115 VAL C, 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C 2.84 44.37 -1.96 -0.65 2.85 86 34 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C 2.92 44.95 -3.5 -1.1 3.53 84 35 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 300119 GLN C 2.9 45.27 2.66 0.68 0.81 95 36 200115 VAL C, 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C 2.99 45.37 -1.96 -0.65 2.85 86 37 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C 2.67 51.39 -3.5 -1.1 3.53 84 38 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C, 300124 HIS C 3.61 52.84 -3.44 -0.83 13.14 85 39 200119 GLN C, 300124 HIS C, 124 HIS C, 100124 HIS C, 200124 HIS C 4.44 53 -3.26 -0.01 41.99 89 40 100119 GLN C, 300124 HIS C, 124 HIS C, 100124 HIS C, 200124 HIS C 4.42 53.25 -3.26 -0.01 41.99 89 41 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C, 300124 HIS C 4.39 53.29 -3.44 -0.83 13.14 85 42 119 GLN C, 200119 GLN C, 300119 GLN C, 300124 HIS C, 124 HIS C 4.38 53.37 -3.38 -0.56 22.76 86 43 100119 GLN C, 200119 GLN C, 300119 GLN C, 100124 HIS C, 200124 HIS C 4.33 53.69 -3.38 -0.56 22.76 86 44 119 GLN C, 200119 GLN C, 300119 GLN C, 300124 HIS C, 124 HIS C 4.18 54.42 -3.38 -0.56 22.76 86 45 100119 GLN C, 200119 GLN C, 300119 GLN C, 100124 HIS C, 200124 HIS C 3.86 56.05 -3.38 -0.56 22.76 86 46 100119 GLN C, 200119 GLN C, 100124 HIS C, 200124 HIS C 2.83 58.08 -3.35 -0.42 27.57 87 47 100119 GLN C, 100124 HIS C, 200124 HIS C 2.39 60.94 -3.3 -0.19 35.58 88 48 100119 GLN C, 100124 HIS C, 100122 ARG C, 200124 HIS C 2.26 61.64 -2.9 -0.52 27.63 86 49 100124 HIS C, 100122 ARG C, 200124 HIS C 2.24 62.25 -2.7 -0.32 35.66 87 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
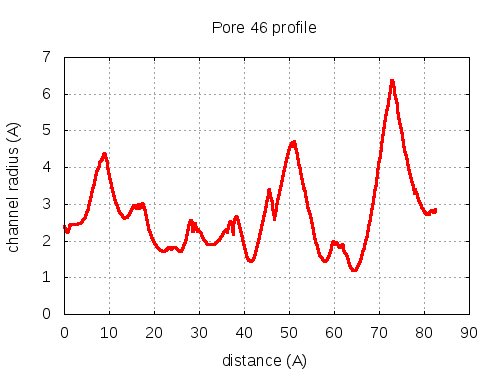
Unique lining residues set - all
100106 VAL C, 100107 THR C, 100110 LEU C, 200101 THR C, 100103 PHE C, 200100 ILE C, 200100 ILE C, 200104 GLY C, 200103 PHE C, 100 ILE C, 200107 THR C, 107 THR C, 100100 ILE C, 300100 ILE C, 300107 THR C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300108 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C, 100124 HIS C, 124 HIS C, 200124 HIS C, 300124 HIS C, 100124 HIS C, 300122 ARG C
Unique lining residues set - sidechains
100106 VAL C, 100107 THR C, 100110 LEU C, 200100 ILE C, 200103 PHE C, 100 ILE C, 200107 THR C, 107 THR C, 100100 ILE C, 300100 ILE C, 300107 THR C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C, 100124 HIS C, 124 HIS C, 200124 HIS C, 300124 HIS C, 300122 ARG C
Physicochemical properties of lining side-chains
Charge: 1 (1-0)
Hydropathy: 0.5
Hydrophobicity: 0
Polarity: 5.61
Mutability: 93
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 100106 VAL C, 100107 THR C, 100110 LEU C, 200101 THR C 1.86 0.61 1.73 0.18 1.33 86 2 100106 VAL C, 100107 THR C, 200101 THR C 1.83 0.88 1.03 -0.15 1.72 102 3 100106 VAL C, 100107 THR C, 200101 THR C, 100103 PHE C 1.85 1.47 0.68 -0.31 2.14 102 4 100106 VAL C, 100107 THR C, 200101 THR C, 100103 PHE C, 200100 ILE C 1.96 1.74 0.46 -0.41 2.39 102 5 100107 THR C, 200101 THR C, 100103 PHE C, 200100 ILE C 1.91 2.6 0.75 -0.14 2.14 105 6 100107 THR C, 100103 PHE C, 200100 ILE C 1.44 5.03 1.13 0.08 1.72 105 7 100107 THR C, 200100 ILE C, 200104 GLY C 1.61 8.14 1.13 0.08 1.72 105 8 200100 ILE C, 200104 GLY C, 200103 PHE C 3.22 8.8 2.3 0.79 1.29 77 9 200100 ILE C, 200104 GLY C, 200103 PHE C, 100 ILE C 3.56 9.29 2.85 1.04 1 85 10 200100 ILE C, 200104 GLY C, 100 ILE C, 200107 THR C 3.82 9.71 1.98 0.51 1.33 104 11 100107 THR C, 200100 ILE C, 200104 GLY C, 100 ILE C, 200107 THR C 4.1 10.5 1.44 0.26 1.39 105 12 100107 THR C, 200100 ILE C, 100 ILE C, 200107 THR C, 107 THR C 4.58 10.79 1.38 0.26 1.05 105 13 100 ILE C, 107 THR C, 100100 ILE C, 300100 ILE C, 300107 THR C 4.62 10.95 2.42 0.78 0.74 104 14 100107 THR C, 200100 ILE C, 100 ILE C, 200107 THR C, 107 THR C 4.7 10.99 1.38 0.26 1.05 105 15 100107 THR C, 200100 ILE C, 200107 THR C, 107 THR C, 300107 THR C 4.7 11.08 0.34 -0.25 1.35 106 16 100107 THR C, 100 ILE C, 200107 THR C, 107 THR C, 300107 THR C 4.71 11.18 0.34 -0.25 1.35 106 17 200100 ILE C, 100 ILE C, 100100 ILE C, 300100 ILE C, 300107 THR C 4.63 12.09 3.46 1.29 0.44 103 18 100107 THR C, 100 ILE C, 200107 THR C, 107 THR C, 300107 THR C 4.55 12.75 0.34 -0.25 1.35 106 19 100107 THR C, 200104 GLY C, 200107 THR C, 107 THR C, 300107 THR C 4.16 13.52 -0.64 -0.78 2 107 20 100107 THR C, 200107 THR C, 107 THR C, 300107 THR C 1.44 23.44 -0.7 -0.77 1.66 107 21 100107 THR C, 200107 THR C, 107 THR C, 300107 THR C, 100111 ALA C 2.47 24.17 -0.2 -0.61 1.33 105 22 100107 THR C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.4 24.42 1.3 -0.14 0.33 101 23 300107 THR C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.18 25.24 1.3 -0.14 0.33 101 24 100107 THR C, 200107 THR C, 107 THR C, 300107 THR C, 300111 ALA C 2.21 26.84 -0.2 -0.61 1.33 105 25 107 THR C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.29 27.89 1.3 -0.14 0.33 101 26 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300108 ALA C 1.95 29.13 1.36 -0.14 0.68 100 27 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 1.79 31.39 1.8 0.02 0 100 28 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300115 VAL C 2.23 32.04 2.28 0.24 0.03 99 29 300111 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 2.22 32.56 3.72 0.91 0.1 98 30 111 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 2.37 33.16 3.72 0.91 0.1 98 31 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300115 VAL C 2.28 33.4 2.28 0.24 0.03 99 32 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 115 VAL C 2.26 34.02 2.28 0.24 0.03 99 33 100111 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 2.15 35.63 3.72 0.91 0.1 98 34 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 1.72 42.66 4.2 1.13 0.13 98 35 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 119 GLN C 2.16 44.26 2.66 0.68 0.81 95 36 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C 2.89 44.91 -3.5 -1.1 3.53 84 37 300115 VAL C, 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C 2.97 45.07 -1.96 -0.65 2.85 86 38 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 300119 GLN C 2.94 45.14 2.66 0.68 0.81 95 39 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 119 GLN C 2.91 45.37 2.66 0.68 0.81 95 40 200115 VAL C, 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C 2.99 45.47 -1.96 -0.65 2.85 86 41 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C 2.65 51.64 -3.5 -1.1 3.53 84 42 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C, 100124 HIS C 3.73 52.96 -3.44 -0.83 13.14 85 43 200119 GLN C, 100124 HIS C, 124 HIS C, 200124 HIS C, 300124 HIS C 4.4 53.33 -3.26 -0.01 41.99 89 44 119 GLN C, 100119 GLN C, 300119 GLN C, 100124 HIS C, 300124 HIS C 4.32 53.69 -3.38 -0.56 22.76 86 45 119 GLN C, 100119 GLN C, 200119 GLN C, 124 HIS C, 200124 HIS C 4.18 54.42 -3.38 -0.56 22.76 86 46 119 GLN C, 100119 GLN C, 300119 GLN C, 100124 HIS C, 300124 HIS C 3.86 56.04 -3.38 -0.56 22.76 86 47 100119 GLN C, 300119 GLN C, 100124 HIS C, 300124 HIS C 2.82 58.07 -3.35 -0.42 27.57 87 48 300119 GLN C, 100124 HIS C, 300124 HIS C 2.39 60.94 -3.3 -0.19 35.58 88 49 300119 GLN C, 300124 HIS C, 100124 HIS C, 300122 ARG C 2.26 61.63 -2.9 -0.52 27.63 86 50 300124 HIS C, 100124 HIS C, 300122 ARG C 2.24 62.24 -2.7 -0.32 35.66 87 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
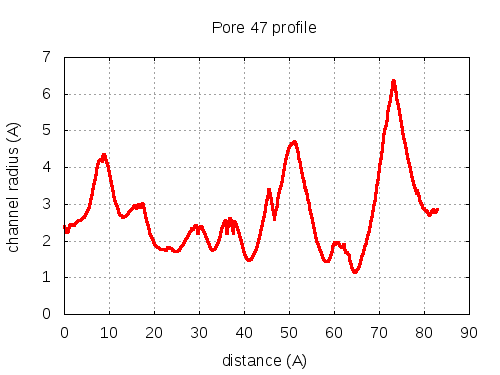
Unique lining residues set - all
100106 VAL C, 100107 THR C, 100110 LEU C, 200101 THR C, 100103 PHE C, 200100 ILE C, 200100 ILE C, 200104 GLY C, 200103 PHE C, 100 ILE C, 200107 THR C, 107 THR C, 100100 ILE C, 300100 ILE C, 300107 THR C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300108 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C, 300124 HIS C, 124 HIS C, 100124 HIS C, 200124 HIS C, 100122 ARG C, 200124 HIS C
Unique lining residues set - sidechains
100106 VAL C, 100107 THR C, 100110 LEU C, 200100 ILE C, 200103 PHE C, 100 ILE C, 200107 THR C, 107 THR C, 100100 ILE C, 300100 ILE C, 300107 THR C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C, 300124 HIS C, 124 HIS C, 100124 HIS C, 200124 HIS C, 100122 ARG C,
Physicochemical properties of lining side-chains
Charge: 1 (1-0)
Hydropathy: 0.3
Hydrophobicity: -0.03
Polarity: 6.36
Mutability: 93
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 100106 VAL C, 100107 THR C, 100110 LEU C, 200101 THR C 1.86 0.63 1.73 0.18 1.33 86 2 100106 VAL C, 100107 THR C, 200101 THR C 1.83 0.9 1.03 -0.15 1.72 102 3 100106 VAL C, 100107 THR C, 200101 THR C, 100103 PHE C 1.85 1.48 0.68 -0.31 2.14 102 4 100106 VAL C, 100107 THR C, 200101 THR C, 100103 PHE C, 200100 ILE C 1.95 1.76 0.46 -0.41 2.39 102 5 100107 THR C, 200101 THR C, 100103 PHE C, 200100 ILE C 1.88 2.75 0.75 -0.14 2.14 105 6 100107 THR C, 100103 PHE C, 200100 ILE C 1.47 4.38 1.13 0.08 1.72 105 7 100107 THR C, 100103 PHE C, 200100 ILE C, 200104 GLY C 1.47 5.35 0.75 -0.14 2.14 105 8 100107 THR C, 200100 ILE C, 200104 GLY C 1.72 8.25 1.13 0.08 1.72 105 9 200100 ILE C, 200104 GLY C, 200103 PHE C 3.29 8.83 2.3 0.79 1.29 77 10 200100 ILE C, 200104 GLY C, 200103 PHE C, 100 ILE C 3.58 9.25 2.85 1.04 1 85 11 200100 ILE C, 200104 GLY C, 100 ILE C, 200107 THR C 3.8 9.67 1.98 0.51 1.33 104 12 100107 THR C, 200100 ILE C, 200104 GLY C, 100 ILE C, 200107 THR C 4.07 10.48 1.44 0.26 1.39 105 13 100107 THR C, 200100 ILE C, 100 ILE C, 200107 THR C, 107 THR C 4.55 10.79 1.38 0.26 1.05 105 14 100 ILE C, 107 THR C, 100100 ILE C, 300100 ILE C, 300107 THR C 4.63 10.94 2.42 0.78 0.74 104 15 100107 THR C, 200100 ILE C, 100 ILE C, 200107 THR C, 107 THR C 4.69 10.99 1.38 0.26 1.05 105 16 100107 THR C, 200100 ILE C, 200107 THR C, 107 THR C, 300107 THR C 4.69 11.08 0.34 -0.25 1.35 106 17 100107 THR C, 100 ILE C, 200107 THR C, 107 THR C, 300107 THR C 4.7 11.2 0.34 -0.25 1.35 106 18 200100 ILE C, 100 ILE C, 100100 ILE C, 300100 ILE C, 300107 THR C 4.65 12.23 3.46 1.29 0.44 103 19 100107 THR C, 200104 GLY C, 200107 THR C, 107 THR C, 300107 THR C 4.09 13.74 -0.64 -0.78 2 107 20 100107 THR C, 200107 THR C, 107 THR C, 300107 THR C 1.54 23.03 -0.7 -0.77 1.66 107 21 100107 THR C, 200107 THR C, 107 THR C, 300107 THR C, 100111 ALA C 2.3 24 -0.2 -0.61 1.33 105 22 100107 THR C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.29 24.46 1.3 -0.14 0.33 101 23 300107 THR C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.17 25.52 1.3 -0.14 0.33 101 24 100107 THR C, 200107 THR C, 107 THR C, 300107 THR C, 300111 ALA C 2.19 26.7 -0.2 -0.61 1.33 105 25 107 THR C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.57 27.7 1.3 -0.14 0.33 101 26 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300108 ALA C 2.09 28.94 1.36 -0.14 0.68 100 27 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 1.75 31.31 1.8 0.02 0 100 28 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300115 VAL C 2.19 32.03 2.28 0.24 0.03 99 29 300111 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 2.23 32.57 3.72 0.91 0.1 98 30 111 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 2.37 33.18 3.72 0.91 0.1 98 31 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300115 VAL C 2.29 33.43 2.28 0.24 0.03 99 32 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 115 VAL C 2.29 34.08 2.28 0.24 0.03 99 33 100111 ALA C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 2.09 35.74 3.72 0.91 0.1 98 34 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 1.69 43.08 4.2 1.13 0.13 98 35 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 119 GLN C 2.4 44.44 2.66 0.68 0.81 95 36 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C 2.91 45.07 -3.5 -1.1 3.53 84 37 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 300119 GLN C 2.9 45.42 2.66 0.68 0.81 95 38 200115 VAL C, 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C 2.99 45.52 -1.96 -0.65 2.85 86 39 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C 2.65 51.73 -3.5 -1.1 3.53 84 40 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C, 300124 HIS C 3.74 53.06 -3.44 -0.83 13.14 85 41 100119 GLN C, 300124 HIS C, 124 HIS C, 100124 HIS C, 200124 HIS C 4.41 53.39 -3.26 -0.01 41.99 89 42 119 GLN C, 100119 GLN C, 200119 GLN C, 300119 GLN C, 300124 HIS C 4.39 53.43 -3.44 -0.83 13.14 85 43 119 GLN C, 200119 GLN C, 300119 GLN C, 300124 HIS C, 124 HIS C 4.38 53.48 -3.38 -0.56 22.76 86 44 100119 GLN C, 200119 GLN C, 300119 GLN C, 100124 HIS C, 200124 HIS C 4.34 53.76 -3.38 -0.56 22.76 86 45 119 GLN C, 200119 GLN C, 300119 GLN C, 300124 HIS C, 124 HIS C 4.16 55.02 -3.38 -0.56 22.76 86 46 100119 GLN C, 200119 GLN C, 300119 GLN C, 100124 HIS C, 200124 HIS C 4.01 55.92 -3.38 -0.56 22.76 86 47 100119 GLN C, 200119 GLN C, 100124 HIS C, 200124 HIS C 2.91 57.99 -3.35 -0.42 27.57 87 48 100119 GLN C, 100124 HIS C, 200124 HIS C 2.39 61.02 -3.3 -0.19 35.58 88 49 100119 GLN C, 100124 HIS C, 100122 ARG C, 200124 HIS C 2.26 61.73 -2.9 -0.52 27.63 86 50 100124 HIS C, 100122 ARG C, 200124 HIS C 2.24 62.36 -2.7 -0.32 35.66 87 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
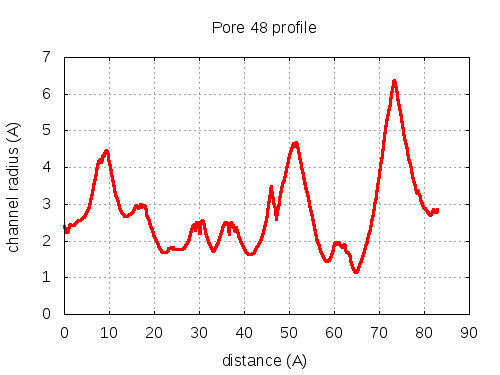
Unique lining residues set - all
122 ARG C, 124 HIS C, 300124 HIS C, 119 GLN C, 300124 HIS C, 300119 GLN C, 200119 GLN C, 100119 GLN C, 100124 HIS C, 200124 HIS C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300108 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 300104 GLY C, 100100 ILE C, 100 ILE C, 200100 ILE C, 300100 ILE C, 300103 PHE C, 103 PHE C, 300101 THR C, 106 VAL C, 300100 ILE C, 110 LEU C, 300101 THR C
Unique lining residues set - sidechains
122 ARG C, 124 HIS C, 119 GLN C, 300124 HIS C, 300119 GLN C, 200119 GLN C, 100119 GLN C, 100124 HIS C, 200124 HIS C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100100 ILE C, 100 ILE C, 200100 ILE C, 300100 ILE C, 300103 PHE C, 106 VAL C, 110 LEU C, 300101 THR C
Physicochemical properties of lining side-chains
Charge: 1 (1-0)
Hydropathy: 0.5
Hydrophobicity: -0.02
Polarity: 5.5
Mutability: 93
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 122 ARG C, 124 HIS C, 300124 HIS C 2.25 0.79 -2.7 -0.32 35.66 87 2 122 ARG C, 124 HIS C, 300124 HIS C, 119 GLN C 2.24 1.71 -2.9 -0.52 27.63 86 3 124 HIS C, 119 GLN C, 300124 HIS C 2.39 5.77 -3.3 -0.19 35.58 88 4 124 HIS C, 119 GLN C, 300124 HIS C, 300119 GLN C 3.05 7.4 -3.35 -0.42 27.57 87 5 124 HIS C, 119 GLN C, 300124 HIS C, 300119 GLN C, 200119 GLN C 4.11 7.95 -3.38 -0.56 22.76 86 6 300119 GLN C, 200119 GLN C, 100119 GLN C, 100124 HIS C, 200124 HIS C 4.31 8.37 -3.38 -0.56 22.76 86 7 124 HIS C, 200119 GLN C, 100119 GLN C, 100124 HIS C, 200124 HIS C 4.16 9.11 -3.32 -0.28 32.37 88 8 119 GLN C, 300124 HIS C, 300119 GLN C, 200119 GLN C, 100119 GLN C 3.73 10.63 -3.44 -0.83 13.14 85 9 119 GLN C, 300119 GLN C, 200119 GLN C, 100119 GLN C 2.65 16.45 -3.5 -1.1 3.53 84 10 119 GLN C, 300119 GLN C, 200119 GLN C, 100119 GLN C, 300115 VAL C 3.01 16.53 -1.96 -0.65 2.85 86 11 100119 GLN C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 2.9 16.82 2.66 0.68 0.81 95 12 119 GLN C, 300119 GLN C, 200119 GLN C, 100119 GLN C, 300115 VAL C 2.98 17 -1.96 -0.65 2.85 86 13 119 GLN C, 300119 GLN C, 200119 GLN C, 100119 GLN C 2.88 18.22 -3.5 -1.1 3.53 84 14 119 GLN C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 2.25 20.01 2.66 0.68 0.81 95 15 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 1.71 27.05 4.2 1.13 0.13 98 16 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 100111 ALA C 2.1 28.19 3.72 0.91 0.1 98 17 115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.24 28.58 2.28 0.24 0.03 99 18 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.32 29.05 2.28 0.24 0.03 99 19 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 111 ALA C 2.49 29.33 3.72 0.91 0.1 98 20 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 100111 ALA C 2.23 30.97 3.72 0.91 0.1 98 21 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.14 31.99 2.28 0.24 0.03 99 22 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 1.74 34.19 1.8 0.02 0 100 23 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300108 ALA C 2.1 35.1 1.36 -0.14 0.68 100 24 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C 2.49 35.71 1.3 -0.14 0.33 101 25 100111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 2.16 36.84 -0.2 -0.61 1.33 105 26 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C 2.22 38.32 1.3 -0.14 0.33 101 27 100111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 2.25 39.34 -0.2 -0.61 1.33 105 28 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 1.54 48.97 -0.7 -0.77 1.66 107 29 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 300104 GLY C 4.05 50.1 -0.64 -0.78 2 107 30 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100100 ILE C 4.76 50.4 0.34 -0.25 1.35 106 31 200107 THR C, 100100 ILE C, 100 ILE C, 200100 ILE C, 300100 ILE C 4.56 50.98 3.46 1.29 0.44 103 32 107 THR C, 100107 THR C, 300107 THR C, 100100 ILE C, 300100 ILE C 4.56 51.41 1.38 0.26 1.05 105 33 107 THR C, 300107 THR C, 300104 GLY C, 100100 ILE C, 300100 ILE C 3.99 52.28 1.44 0.26 1.39 105 34 300107 THR C, 300104 GLY C, 100100 ILE C, 300100 ILE C 3.87 52.43 1.98 0.51 1.33 104 35 300104 GLY C, 100100 ILE C, 300100 ILE C, 300103 PHE C 3.55 52.85 2.85 1.04 1 85 36 300104 GLY C, 300100 ILE C, 300103 PHE C 3.17 53.84 2.3 0.79 1.29 77 37 107 THR C, 300104 GLY C, 300100 ILE C 1.7 57.17 1.13 0.08 1.72 105 38 107 THR C, 300104 GLY C, 300100 ILE C, 103 PHE C 1.47 57.97 0.75 -0.14 2.14 105 39 107 THR C, 300100 ILE C, 103 PHE C 1.45 59.44 1.13 0.08 1.72 105 40 107 THR C, 300100 ILE C, 103 PHE C, 300101 THR C 1.91 60.04 0.75 -0.14 2.14 105 41 107 THR C, 103 PHE C, 300101 THR C, 106 VAL C, 300100 ILE C 1.96 60.18 0.46 -0.41 2.39 102 42 107 THR C, 103 PHE C, 300101 THR C, 106 VAL C 1.84 61.09 0.68 -0.31 2.14 102 43 107 THR C, 300101 THR C, 106 VAL C 1.83 61.35 1.03 -0.15 1.72 102 44 107 THR C, 300101 THR C, 106 VAL C, 110 LEU C 1.8 61.96 1.73 0.18 1.33 86 45 106 VAL C, 110 LEU C, 300101 THR C 1.22 66.38 2.43 0.5 0.64 86 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
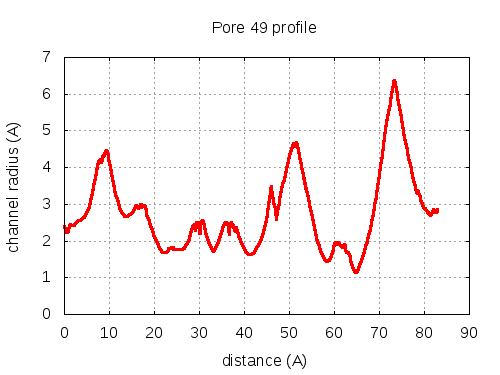
Unique lining residues set - all
122 ARG C, 124 HIS C, 300124 HIS C, 119 GLN C, 300124 HIS C, 300119 GLN C, 200119 GLN C, 100119 GLN C, 100124 HIS C, 200124 HIS C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300108 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100104 GLY C, 100100 ILE C, 100 ILE C, 200100 ILE C, 300100 ILE C, 104 GLY C, 103 PHE C, 200103 PHE C, 101 THR C, 100 ILE C, 200106 VAL C, 200110 LEU C, 101 THR C
Unique lining residues set - sidechains
122 ARG C, 124 HIS C, 119 GLN C, 300124 HIS C, 300119 GLN C, 200119 GLN C, 100119 GLN C, 100124 HIS C, 200124 HIS C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100100 ILE C, 100 ILE C, 200100 ILE C, 300100 ILE C, 103 PHE C, 200106 VAL C, 200110 LEU C, 101 THR C
Physicochemical properties of lining side-chains
Charge: 1 (1-0)
Hydropathy: 0.4
Hydrophobicity: -0.03
Polarity: 5.23
Mutability: 93
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 122 ARG C, 124 HIS C, 300124 HIS C 2.25 0.81 -2.7 -0.32 35.66 87 2 122 ARG C, 124 HIS C, 300124 HIS C, 119 GLN C 2.24 1.76 -2.9 -0.52 27.63 86 3 124 HIS C, 119 GLN C, 300124 HIS C 2.38 5.09 -3.3 -0.19 35.58 88 4 124 HIS C, 119 GLN C, 300124 HIS C, 300119 GLN C 2.79 6.9 -3.35 -0.42 27.57 87 5 124 HIS C, 119 GLN C, 300124 HIS C, 300119 GLN C, 200119 GLN C 3.7 8.09 -3.38 -0.56 22.76 86 6 300119 GLN C, 200119 GLN C, 100119 GLN C, 100124 HIS C, 200124 HIS C 4.27 8.38 -3.38 -0.56 22.76 86 7 124 HIS C, 200119 GLN C, 100119 GLN C, 100124 HIS C, 200124 HIS C 4.18 8.93 -3.32 -0.28 32.37 88 8 119 GLN C, 300124 HIS C, 300119 GLN C, 200119 GLN C, 100119 GLN C 3.55 10.99 -3.44 -0.83 13.14 85 9 119 GLN C, 300119 GLN C, 200119 GLN C, 100119 GLN C 2.62 16.45 -3.5 -1.1 3.53 84 10 119 GLN C, 300119 GLN C, 200119 GLN C, 100119 GLN C, 300115 VAL C 2.99 16.54 -1.96 -0.65 2.85 86 11 100119 GLN C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 2.9 16.84 2.66 0.68 0.81 95 12 119 GLN C, 300119 GLN C, 200119 GLN C, 100119 GLN C, 300115 VAL C 3 17.03 -1.96 -0.65 2.85 86 13 119 GLN C, 300119 GLN C, 200119 GLN C, 100119 GLN C 2.91 17.75 -3.5 -1.1 3.53 84 14 119 GLN C, 300119 GLN C, 200119 GLN C, 100119 GLN C, 300115 VAL C 2.88 18.43 -1.96 -0.65 2.85 86 15 119 GLN C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 2.6 19.33 2.66 0.68 0.81 95 16 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 1.72 27.5 4.2 1.13 0.13 98 17 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 100111 ALA C 2.35 28.05 3.72 0.91 0.1 98 18 115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.25 28.52 2.28 0.24 0.03 99 19 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.29 28.98 2.28 0.24 0.03 99 20 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 111 ALA C 2.39 29.49 3.72 0.91 0.1 98 21 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 300111 ALA C 2.23 30.9 3.72 0.91 0.1 98 22 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.1 31.93 2.28 0.24 0.03 99 23 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 1.77 34.18 1.8 0.02 0 100 24 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300108 ALA C 2.02 35.1 1.36 -0.14 0.68 100 25 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C 2.43 35.71 1.3 -0.14 0.33 101 26 300111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 2.17 36.86 -0.2 -0.61 1.33 105 27 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300107 THR C 2.18 38.48 1.3 -0.14 0.33 101 28 100111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 2.35 39.61 -0.2 -0.61 1.33 105 29 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 1.49 49.41 -0.7 -0.77 1.66 107 30 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100104 GLY C 4.38 49.97 -0.64 -0.78 2 107 31 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100100 ILE C 4.62 50.32 0.34 -0.25 1.35 106 32 107 THR C, 100100 ILE C, 100 ILE C, 200100 ILE C, 300100 ILE C 4.64 50.8 3.46 1.29 0.44 103 33 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100100 ILE C 4.69 50.86 0.34 -0.25 1.35 106 34 100107 THR C, 200107 THR C, 300107 THR C, 100100 ILE C, 200100 ILE C 4.63 51.06 1.38 0.26 1.05 105 35 107 THR C, 200107 THR C, 300107 THR C, 100 ILE C, 300100 ILE C 4.59 51.44 1.38 0.26 1.05 105 36 107 THR C, 200107 THR C, 100 ILE C, 300100 ILE C, 104 GLY C 4.01 52.36 1.44 0.26 1.39 105 37 107 THR C, 100 ILE C, 300100 ILE C, 104 GLY C 3.78 52.65 1.98 0.51 1.33 104 38 100 ILE C, 300100 ILE C, 104 GLY C, 103 PHE C 3.56 52.98 2.85 1.04 1 85 39 100 ILE C, 104 GLY C, 103 PHE C 3.29 53.75 2.3 0.79 1.29 77 40 200107 THR C, 100 ILE C, 104 GLY C 1.6 57.62 1.13 0.08 1.72 105 41 200107 THR C, 100 ILE C, 104 GLY C, 200103 PHE C 1.43 58.41 0.75 -0.14 2.14 105 42 200107 THR C, 100 ILE C, 200103 PHE C 1.49 59.44 1.13 0.08 1.72 105 43 200107 THR C, 100 ILE C, 200103 PHE C, 101 THR C 1.87 60.13 0.75 -0.14 2.14 105 44 200107 THR C, 200103 PHE C, 101 THR C, 100 ILE C, 200106 VAL C 1.95 60.42 0.46 -0.41 2.39 102 45 200107 THR C, 200103 PHE C, 101 THR C, 200106 VAL C 1.85 61.15 0.68 -0.31 2.14 102 46 200107 THR C, 101 THR C, 200106 VAL C 1.83 61.42 1.03 -0.15 1.72 102 47 200107 THR C, 101 THR C, 200106 VAL C, 200110 LEU C 1.74 62.26 1.73 0.18 1.33 86 48 200106 VAL C, 200110 LEU C, 101 THR C 1.23 66.52 2.43 0.5 0.64 86 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
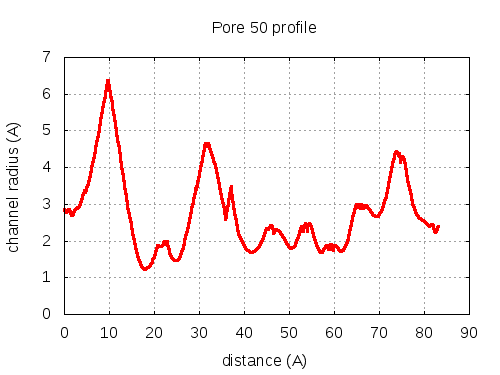
Unique lining residues set - all
122 ARG C, 124 HIS C, 300124 HIS C, 119 GLN C, 300124 HIS C, 300119 GLN C, 200119 GLN C, 100119 GLN C, 100124 HIS C, 200124 HIS C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300108 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 104 GLY C, 100 ILE C, 100100 ILE C, 200100 ILE C, 300100 ILE C, 100104 GLY C, 100103 PHE C, 300103 PHE C, 100101 THR C, 100100 ILE C, 300106 VAL C, 300110 LEU C, 100101 THR C
Unique lining residues set - sidechains
122 ARG C, 124 HIS C, 119 GLN C, 300124 HIS C, 300119 GLN C, 200119 GLN C, 100119 GLN C, 100124 HIS C, 200124 HIS C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100 ILE C, 100100 ILE C, 200100 ILE C, 300100 ILE C, 100103 PHE C, 300106 VAL C, 300110 LEU C, 100101 THR C
Physicochemical properties of lining side-chains
Charge: 1 (1-0)
Hydropathy: 0.4
Hydrophobicity: -0.03
Polarity: 5.23
Mutability: 93
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 122 ARG C, 124 HIS C, 300124 HIS C 2.25 0.81 -2.7 -0.32 35.66 87 2 122 ARG C, 124 HIS C, 300124 HIS C, 119 GLN C 2.24 1.76 -2.9 -0.52 27.63 86 3 124 HIS C, 119 GLN C, 300124 HIS C 2.38 5.09 -3.3 -0.19 35.58 88 4 124 HIS C, 119 GLN C, 300124 HIS C, 300119 GLN C 2.79 6.9 -3.35 -0.42 27.57 87 5 124 HIS C, 119 GLN C, 300124 HIS C, 300119 GLN C, 200119 GLN C 3.7 8.09 -3.38 -0.56 22.76 86 6 300119 GLN C, 200119 GLN C, 100119 GLN C, 100124 HIS C, 200124 HIS C 4.27 8.38 -3.38 -0.56 22.76 86 7 124 HIS C, 200119 GLN C, 100119 GLN C, 100124 HIS C, 200124 HIS C 4.18 8.93 -3.32 -0.28 32.37 88 8 119 GLN C, 300124 HIS C, 300119 GLN C, 200119 GLN C, 100119 GLN C 3.55 10.99 -3.44 -0.83 13.14 85 9 119 GLN C, 300119 GLN C, 200119 GLN C, 100119 GLN C 2.62 16.45 -3.5 -1.1 3.53 84 10 119 GLN C, 300119 GLN C, 200119 GLN C, 100119 GLN C, 300115 VAL C 2.99 16.54 -1.96 -0.65 2.85 86 11 100119 GLN C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 2.9 16.84 2.66 0.68 0.81 95 12 119 GLN C, 300119 GLN C, 200119 GLN C, 100119 GLN C, 300115 VAL C 3 17.03 -1.96 -0.65 2.85 86 13 119 GLN C, 300119 GLN C, 200119 GLN C, 100119 GLN C 2.91 17.75 -3.5 -1.1 3.53 84 14 119 GLN C, 300119 GLN C, 200119 GLN C, 100119 GLN C, 300115 VAL C 2.88 18.43 -1.96 -0.65 2.85 86 15 119 GLN C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 2.6 19.33 2.66 0.68 0.81 95 16 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 1.72 27.5 4.2 1.13 0.13 98 17 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 100111 ALA C 2.35 28.05 3.72 0.91 0.1 98 18 115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.25 28.52 2.28 0.24 0.03 99 19 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.29 28.98 2.28 0.24 0.03 99 20 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 111 ALA C 2.39 29.49 3.72 0.91 0.1 98 21 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 300111 ALA C 2.23 30.9 3.72 0.91 0.1 98 22 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.1 31.93 2.28 0.24 0.03 99 23 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 1.77 34.18 1.8 0.02 0 100 24 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300108 ALA C 2.02 35.1 1.36 -0.14 0.68 100 25 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C 2.43 35.71 1.3 -0.14 0.33 101 26 300111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 2.17 36.86 -0.2 -0.61 1.33 105 27 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C 2.18 38.48 1.3 -0.14 0.33 101 28 100111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 2.35 39.61 -0.2 -0.61 1.33 105 29 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 1.49 49.41 -0.7 -0.77 1.66 107 30 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 104 GLY C 4.38 49.97 -0.64 -0.78 2 107 31 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100 ILE C 4.62 50.32 0.34 -0.25 1.35 106 32 100107 THR C, 100 ILE C, 100100 ILE C, 200100 ILE C, 300100 ILE C 4.64 50.8 3.46 1.29 0.44 103 33 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100 ILE C 4.69 50.86 0.34 -0.25 1.35 106 34 107 THR C, 200107 THR C, 300107 THR C, 100 ILE C, 300100 ILE C 4.63 51.06 1.38 0.26 1.05 105 35 100107 THR C, 200107 THR C, 300107 THR C, 100100 ILE C, 200100 ILE C 4.59 51.44 1.38 0.26 1.05 105 36 100107 THR C, 300107 THR C, 100100 ILE C, 200100 ILE C, 100104 GLY C 4.01 52.36 1.44 0.26 1.39 105 37 100107 THR C, 100100 ILE C, 200100 ILE C, 100104 GLY C 3.78 52.65 1.98 0.51 1.33 104 38 100100 ILE C, 200100 ILE C, 100104 GLY C, 100103 PHE C 3.56 52.98 2.85 1.04 1 85 39 100100 ILE C, 100104 GLY C, 100103 PHE C 3.29 53.75 2.3 0.79 1.29 77 40 300107 THR C, 100100 ILE C, 100104 GLY C 1.6 57.62 1.13 0.08 1.72 105 41 300107 THR C, 100100 ILE C, 100104 GLY C, 300103 PHE C 1.43 58.41 0.75 -0.14 2.14 105 42 300107 THR C, 100100 ILE C, 300103 PHE C 1.49 59.44 1.13 0.08 1.72 105 43 300107 THR C, 100100 ILE C, 300103 PHE C, 100101 THR C 1.87 60.13 0.75 -0.14 2.14 105 44 300107 THR C, 300103 PHE C, 100101 THR C, 100100 ILE C, 300106 VAL C 1.95 60.42 0.46 -0.41 2.39 102 45 300107 THR C, 300103 PHE C, 100101 THR C, 300106 VAL C 1.85 61.15 0.68 -0.31 2.14 102 46 300107 THR C, 100101 THR C, 300106 VAL C 1.83 61.42 1.03 -0.15 1.72 102 47 300107 THR C, 100101 THR C, 300106 VAL C, 300110 LEU C 1.74 62.26 1.73 0.18 1.33 86 48 300106 VAL C, 300110 LEU C, 100101 THR C 1.23 66.52 2.43 0.5 0.64 86 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
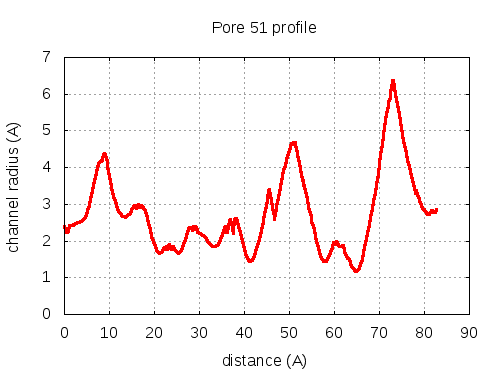
Unique lining residues set - all
122 ARG C, 124 HIS C, 300124 HIS C, 119 GLN C, 300124 HIS C, 300119 GLN C, 200119 GLN C, 100119 GLN C, 100124 HIS C, 200124 HIS C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 200104 GLY C, 100 ILE C, 100100 ILE C, 200100 ILE C, 300100 ILE C, 200103 PHE C, 100103 PHE C, 200101 THR C, 200100 ILE C, 100106 VAL C, 100110 LEU C, 200101 THR C
Unique lining residues set - sidechains
122 ARG C, 124 HIS C, 119 GLN C, 300124 HIS C, 300119 GLN C, 200119 GLN C, 100119 GLN C, 100124 HIS C, 200124 HIS C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100 ILE C, 100100 ILE C, 200100 ILE C, 300100 ILE C, 200103 PHE C, 100106 VAL C, 100110 LEU C, 200101 THR C
Physicochemical properties of lining side-chains
Charge: 1 (1-0)
Hydropathy: 0.5
Hydrophobicity: -0.01
Polarity: 5.13
Mutability: 93
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 122 ARG C, 124 HIS C, 300124 HIS C 2.25 0.82 -2.7 -0.32 35.66 87 2 122 ARG C, 124 HIS C, 300124 HIS C, 119 GLN C 2.25 1.83 -2.9 -0.52 27.63 86 3 124 HIS C, 119 GLN C, 300124 HIS C 2.37 5.39 -3.3 -0.19 35.58 88 4 124 HIS C, 119 GLN C, 300124 HIS C, 300119 GLN C 2.9 7.18 -3.35 -0.42 27.57 87 5 124 HIS C, 119 GLN C, 300124 HIS C, 300119 GLN C, 200119 GLN C 3.88 8.21 -3.38 -0.56 22.76 86 6 300119 GLN C, 200119 GLN C, 100119 GLN C, 100124 HIS C, 200124 HIS C 4.24 8.36 -3.38 -0.56 22.76 86 7 124 HIS C, 200119 GLN C, 100119 GLN C, 100124 HIS C, 200124 HIS C 4.21 9.11 -3.32 -0.28 32.37 88 8 119 GLN C, 300124 HIS C, 300119 GLN C, 200119 GLN C, 100119 GLN C 3.7 10.74 -3.44 -0.83 13.14 85 9 119 GLN C, 300119 GLN C, 200119 GLN C, 100119 GLN C 2.64 16.46 -3.5 -1.1 3.53 84 10 119 GLN C, 300119 GLN C, 200119 GLN C, 100119 GLN C, 300115 VAL C 3 16.55 -1.96 -0.65 2.85 86 11 100119 GLN C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 2.9 16.9 2.66 0.68 0.81 95 12 119 GLN C, 300119 GLN C, 200119 GLN C, 100119 GLN C, 300115 VAL C 2.97 17.1 -1.96 -0.65 2.85 86 13 119 GLN C, 300119 GLN C, 200119 GLN C, 100119 GLN C 2.89 18 -3.5 -1.1 3.53 84 14 119 GLN C, 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 2.35 19.81 2.66 0.68 0.81 95 15 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C 1.73 27.44 4.2 1.13 0.13 98 16 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 100111 ALA C 2.35 28.01 3.72 0.91 0.1 98 17 115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.25 28.49 2.28 0.24 0.03 99 18 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.3 28.98 2.28 0.24 0.03 99 19 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 111 ALA C 2.36 29.48 3.72 0.91 0.1 98 20 300115 VAL C, 115 VAL C, 100115 VAL C, 200115 VAL C, 300111 ALA C 2.22 30.25 3.72 0.91 0.1 98 21 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.01 32.23 2.28 0.24 0.03 99 22 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 1.79 34.5 1.8 0.02 0 100 23 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C 2.11 35.34 1.3 -0.14 0.33 101 24 300111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 2.23 36.67 -0.2 -0.61 1.33 105 25 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300107 THR C 2.18 37.64 1.3 -0.14 0.33 101 26 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 100107 THR C 2.31 39.27 1.3 -0.14 0.33 101 27 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 1.55 48.87 -0.7 -0.77 1.66 107 28 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 200104 GLY C 4 50.09 -0.64 -0.78 2 107 29 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100 ILE C 4.63 50.41 0.34 -0.25 1.35 106 30 300107 THR C, 100 ILE C, 100100 ILE C, 200100 ILE C, 300100 ILE C 4.64 50.9 3.46 1.29 0.44 103 31 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100 ILE C 4.71 50.93 0.34 -0.25 1.35 106 32 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 200100 ILE C 4.69 51.02 0.34 -0.25 1.35 106 33 107 THR C, 100107 THR C, 200107 THR C, 100 ILE C, 200100 ILE C 4.7 51.07 1.38 0.26 1.05 105 34 107 THR C, 300107 THR C, 100 ILE C, 100100 ILE C, 300100 ILE C 4.63 51.35 2.42 0.78 0.74 104 35 107 THR C, 100107 THR C, 200107 THR C, 100 ILE C, 200100 ILE C 4.63 51.55 1.38 0.26 1.05 105 36 100107 THR C, 200107 THR C, 200104 GLY C, 100 ILE C, 200100 ILE C 4.09 52.51 1.44 0.26 1.39 105 37 200107 THR C, 200104 GLY C, 100 ILE C, 200100 ILE C 3.82 52.83 1.98 0.51 1.33 104 38 200104 GLY C, 100 ILE C, 200100 ILE C, 200103 PHE C 3.57 53.19 2.85 1.04 1 85 39 200104 GLY C, 200100 ILE C, 200103 PHE C 3.27 54.01 2.3 0.79 1.29 77 40 100107 THR C, 200104 GLY C, 200100 ILE C 1.86 57.21 1.13 0.08 1.72 105 41 100107 THR C, 200104 GLY C, 200100 ILE C, 100103 PHE C 1.53 58.08 0.75 -0.14 2.14 105 42 100107 THR C, 200100 ILE C, 100103 PHE C 1.43 59.76 1.13 0.08 1.72 105 43 100107 THR C, 200100 ILE C, 100103 PHE C, 200101 THR C 1.91 60.29 0.75 -0.14 2.14 105 44 100107 THR C, 100103 PHE C, 200101 THR C, 200100 ILE C 1.94 60.42 -0.48 -0.79 2.95 107 45 100107 THR C, 100103 PHE C, 200101 THR C, 200100 ILE C, 100106 VAL C 1.97 60.57 0.46 -0.41 2.39 102 46 100107 THR C, 100103 PHE C, 200101 THR C, 100106 VAL C 1.83 61.56 0.68 -0.31 2.14 102 47 100107 THR C, 200101 THR C, 100106 VAL C 1.84 61.81 1.03 -0.15 1.72 102 48 100107 THR C, 200101 THR C, 100106 VAL C, 100110 LEU C 1.76 62.44 1.73 0.18 1.33 86 49 100106 VAL C, 100110 LEU C, 200101 THR C 1.24 66.74 2.43 0.5 0.64 86 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
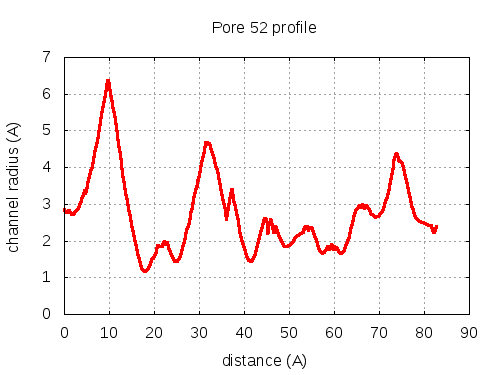
Unique lining residues set - all
124 HIS C, 200122 ARG C, 200124 HIS C, 200119 GLN C, 124 HIS C, 119 GLN C, 100119 GLN C, 100124 HIS C, 300119 GLN C, 300124 HIS C, 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300108 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 300104 GLY C, 100100 ILE C, 100 ILE C, 200100 ILE C, 300100 ILE C, 300103 PHE C, 103 PHE C, 300101 THR C, 106 VAL C, 300100 ILE C, 110 LEU C, 300101 THR C
Unique lining residues set - sidechains
200122 ARG C, 200124 HIS C, 200119 GLN C, 124 HIS C, 119 GLN C, 100119 GLN C, 100124 HIS C, 300119 GLN C, 300124 HIS C, 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100100 ILE C, 100 ILE C, 200100 ILE C, 300100 ILE C, 300103 PHE C, 106 VAL C, 110 LEU C, 300101 THR C
Physicochemical properties of lining side-chains
Charge: 1 (1-0)
Hydropathy: 0.3
Hydrophobicity: -0.02
Polarity: 6.06
Mutability: 93
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 124 HIS C, 200122 ARG C, 200124 HIS C 2.25 0.82 -2.7 -0.32 35.66 87 2 124 HIS C, 200122 ARG C, 200124 HIS C, 200119 GLN C 2.25 1.81 -2.9 -0.52 27.63 86 3 200124 HIS C, 200119 GLN C, 124 HIS C 2.37 5.34 -3.3 -0.19 35.58 88 4 200124 HIS C, 200119 GLN C, 124 HIS C, 119 GLN C 2.88 7.13 -3.35 -0.42 27.57 87 5 200124 HIS C, 200119 GLN C, 124 HIS C, 119 GLN C, 100119 GLN C 3.86 8.17 -3.38 -0.56 22.76 86 6 119 GLN C, 100119 GLN C, 100124 HIS C, 300119 GLN C, 300124 HIS C 4.18 8.77 -3.38 -0.56 22.76 86 7 200124 HIS C, 200119 GLN C, 124 HIS C, 119 GLN C, 100119 GLN C 4.33 9 -3.38 -0.56 22.76 86 8 200124 HIS C, 124 HIS C, 100124 HIS C, 300119 GLN C, 300124 HIS C 4.41 9.5 -3.26 -0.01 41.99 89 9 200119 GLN C, 124 HIS C, 119 GLN C, 100119 GLN C, 300119 GLN C 3.87 11.02 -3.44 -0.83 13.14 85 10 200119 GLN C, 119 GLN C, 100119 GLN C, 300119 GLN C 2.65 16.89 -3.5 -1.1 3.53 84 11 200119 GLN C, 119 GLN C, 100119 GLN C, 300119 GLN C, 200115 VAL C 3 16.98 -1.96 -0.65 2.85 86 12 300119 GLN C, 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C 2.91 17.29 2.66 0.68 0.81 95 13 200119 GLN C, 119 GLN C, 100119 GLN C, 300119 GLN C, 300115 VAL C 2.97 17.48 -1.96 -0.65 2.85 86 14 200119 GLN C, 119 GLN C, 100119 GLN C, 300119 GLN C 2.9 18.23 -3.5 -1.1 3.53 84 15 119 GLN C, 300119 GLN C, 200115 VAL C, 115 VAL C, 300115 VAL C 3.01 18.93 1.12 0.24 1.49 92 16 119 GLN C, 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C 2.58 19.86 2.66 0.68 0.81 95 17 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C 1.71 27.59 4.2 1.13 0.13 98 18 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C, 100111 ALA C 2.15 28.73 3.72 0.91 0.1 98 19 115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.26 29.08 2.28 0.24 0.03 99 20 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.35 29.61 2.28 0.24 0.03 99 21 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C, 100111 ALA C 2.21 31.05 3.72 0.91 0.1 98 22 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.18 32.04 2.28 0.24 0.03 99 23 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 1.79 34.34 1.8 0.02 0 100 24 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300108 ALA C 1.93 35.35 1.36 -0.14 0.68 100 25 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C 2.24 36.05 1.3 -0.14 0.33 101 26 100111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 2.19 37.28 -0.2 -0.61 1.33 105 27 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C 2.19 38.95 1.3 -0.14 0.33 101 28 100111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 2.44 40.11 -0.2 -0.61 1.33 105 29 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 1.44 49.43 -0.7 -0.77 1.66 107 30 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 300104 GLY C 4.06 50.58 -0.64 -0.78 2 107 31 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100100 ILE C 4.7 50.85 0.34 -0.25 1.35 106 32 200107 THR C, 100100 ILE C, 100 ILE C, 200100 ILE C, 300100 ILE C 4.57 51.3 3.46 1.29 0.44 103 33 100107 THR C, 200107 THR C, 100100 ILE C, 100 ILE C, 200100 ILE C 4.65 51.49 2.42 0.78 0.74 104 34 107 THR C, 100107 THR C, 300107 THR C, 100100 ILE C, 300100 ILE C 4.62 51.7 1.38 0.26 1.05 105 35 107 THR C, 300107 THR C, 300104 GLY C, 100100 ILE C, 300100 ILE C 4.05 52.64 1.44 0.26 1.39 105 36 300107 THR C, 300104 GLY C, 100100 ILE C, 300100 ILE C 3.81 52.95 1.98 0.51 1.33 104 37 300104 GLY C, 100100 ILE C, 300100 ILE C, 300103 PHE C 3.56 53.28 2.85 1.04 1 85 38 300104 GLY C, 300100 ILE C, 300103 PHE C 3.25 54.13 2.3 0.79 1.29 77 39 107 THR C, 300104 GLY C, 300100 ILE C 1.8 57.41 1.13 0.08 1.72 105 40 107 THR C, 300104 GLY C, 300100 ILE C, 103 PHE C 1.5 58.27 0.75 -0.14 2.14 105 41 107 THR C, 300100 ILE C, 103 PHE C 1.44 59.86 1.13 0.08 1.72 105 42 107 THR C, 300100 ILE C, 103 PHE C, 300101 THR C 1.91 60.37 0.75 -0.14 2.14 105 43 107 THR C, 103 PHE C, 300101 THR C, 106 VAL C, 300100 ILE C 1.95 60.65 0.46 -0.41 2.39 102 44 107 THR C, 103 PHE C, 300101 THR C, 106 VAL C 1.87 61.37 0.68 -0.31 2.14 102 45 107 THR C, 300101 THR C, 106 VAL C 1.83 61.89 1.03 -0.15 1.72 102 46 107 THR C, 300101 THR C, 106 VAL C, 110 LEU C 1.75 62.52 1.73 0.18 1.33 86 47 106 VAL C, 110 LEU C, 300101 THR C 1.24 66.81 2.43 0.5 0.64 86 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
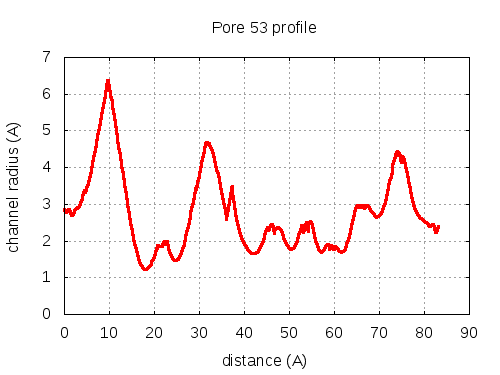
Unique lining residues set - all
124 HIS C, 200122 ARG C, 200124 HIS C, 200119 GLN C, 124 HIS C, 119 GLN C, 100119 GLN C, 100124 HIS C, 300119 GLN C, 300124 HIS C, 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100104 GLY C, 100100 ILE C, 100 ILE C, 200100 ILE C, 300100 ILE C, 104 GLY C, 103 PHE C, 200103 PHE C, 101 THR C, 100 ILE C, 200106 VAL C, 200110 LEU C, 101 THR C
Unique lining residues set - sidechains
200122 ARG C, 200124 HIS C, 200119 GLN C, 124 HIS C, 119 GLN C, 100119 GLN C, 100124 HIS C, 300119 GLN C, 300124 HIS C, 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100100 ILE C, 100 ILE C, 200100 ILE C, 300100 ILE C, 103 PHE C, 200106 VAL C, 200110 LEU C, 101 THR C
Physicochemical properties of lining side-chains
Charge: 1 (1-0)
Hydropathy: 0.4
Hydrophobicity: -0.01
Polarity: 6.13
Mutability: 93
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 124 HIS C, 200122 ARG C, 200124 HIS C 2.25 0.83 -2.7 -0.32 35.66 87 2 124 HIS C, 200122 ARG C, 200124 HIS C, 200119 GLN C 2.25 1.89 -2.9 -0.52 27.63 86 3 200124 HIS C, 200119 GLN C, 124 HIS C 2.37 5.63 -3.3 -0.19 35.58 88 4 200124 HIS C, 200119 GLN C, 124 HIS C, 119 GLN C 3 7.4 -3.35 -0.42 27.57 87 5 200124 HIS C, 200119 GLN C, 124 HIS C, 119 GLN C, 100119 GLN C 4.04 7.97 -3.38 -0.56 22.76 86 6 119 GLN C, 100119 GLN C, 100124 HIS C, 300119 GLN C, 300124 HIS C 4.16 8.72 -3.38 -0.56 22.76 86 7 200124 HIS C, 200119 GLN C, 124 HIS C, 119 GLN C, 100119 GLN C 4.33 8.97 -3.38 -0.56 22.76 86 8 200124 HIS C, 124 HIS C, 100124 HIS C, 300119 GLN C, 300124 HIS C 4.4 9.43 -3.26 -0.01 41.99 89 9 200119 GLN C, 124 HIS C, 119 GLN C, 100119 GLN C, 300119 GLN C 3.54 11.64 -3.44 -0.83 13.14 85 10 200119 GLN C, 119 GLN C, 100119 GLN C, 300119 GLN C 2.67 16.92 -3.5 -1.1 3.53 84 11 200119 GLN C, 119 GLN C, 100119 GLN C, 300119 GLN C, 200115 VAL C 3 17.01 -1.96 -0.65 2.85 86 12 300119 GLN C, 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C 2.9 17.38 2.66 0.68 0.81 95 13 200119 GLN C, 119 GLN C, 100119 GLN C, 300119 GLN C 2.91 18.62 -3.5 -1.1 3.53 84 14 119 GLN C, 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C 2.2 20.54 2.66 0.68 0.81 95 15 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C 1.7 27.63 4.2 1.13 0.13 98 16 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C, 100111 ALA C 2.19 28.72 3.72 0.91 0.1 98 17 115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.27 28.91 2.28 0.24 0.03 99 18 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.27 29.65 2.28 0.24 0.03 99 19 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C, 111 ALA C 2.39 29.9 3.72 0.91 0.1 98 20 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C, 300111 ALA C 2.2 31.44 3.72 0.91 0.1 98 21 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.15 32.54 2.28 0.24 0.03 99 22 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 1.77 34.87 1.8 0.02 0 100 23 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C 2.15 36.27 1.3 -0.14 0.33 101 24 300111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 2.21 37.15 -0.2 -0.61 1.33 105 25 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300107 THR C 2.24 38.8 1.3 -0.14 0.33 101 26 100111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 2.18 39.92 -0.2 -0.61 1.33 105 27 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 1.6 49.65 -0.7 -0.77 1.66 107 28 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100104 GLY C 4.25 50.28 -0.64 -0.78 2 107 29 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100100 ILE C 4.62 50.72 0.34 -0.25 1.35 106 30 107 THR C, 100100 ILE C, 100 ILE C, 200100 ILE C, 300100 ILE C 4.62 51.27 3.46 1.29 0.44 103 31 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100100 ILE C 4.7 51.32 0.34 -0.25 1.35 106 32 100107 THR C, 200107 THR C, 300107 THR C, 100100 ILE C, 200100 ILE C 4.65 51.55 1.38 0.26 1.05 105 33 107 THR C, 200107 THR C, 300107 THR C, 100 ILE C, 300100 ILE C 4.54 51.98 1.38 0.26 1.05 105 34 107 THR C, 200107 THR C, 100 ILE C, 300100 ILE C, 104 GLY C 4.09 52.73 1.44 0.26 1.39 105 35 107 THR C, 100 ILE C, 300100 ILE C, 104 GLY C 3.83 53.06 1.98 0.51 1.33 104 36 100 ILE C, 300100 ILE C, 104 GLY C, 103 PHE C 3.57 53.44 2.85 1.04 1 85 37 100 ILE C, 104 GLY C, 103 PHE C 3.22 54.36 2.3 0.79 1.29 77 38 200107 THR C, 100 ILE C, 104 GLY C 1.68 57.86 1.13 0.08 1.72 105 39 200107 THR C, 100 ILE C, 104 GLY C, 200103 PHE C 1.47 58.7 0.75 -0.14 2.14 105 40 200107 THR C, 100 ILE C, 200103 PHE C 1.46 59.86 1.13 0.08 1.72 105 41 200107 THR C, 100 ILE C, 200103 PHE C, 101 THR C 1.85 60.62 0.75 -0.14 2.14 105 42 200107 THR C, 200103 PHE C, 101 THR C, 100 ILE C, 200106 VAL C 1.96 60.76 0.46 -0.41 2.39 102 43 200107 THR C, 200103 PHE C, 101 THR C, 200106 VAL C 1.84 61.74 0.68 -0.31 2.14 102 44 200107 THR C, 101 THR C, 200106 VAL C 1.83 62 1.03 -0.15 1.72 102 45 200107 THR C, 101 THR C, 200106 VAL C, 200110 LEU C 1.77 62.65 1.73 0.18 1.33 86 46 200106 VAL C, 200110 LEU C, 101 THR C 1.24 66.98 2.43 0.5 0.64 86 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
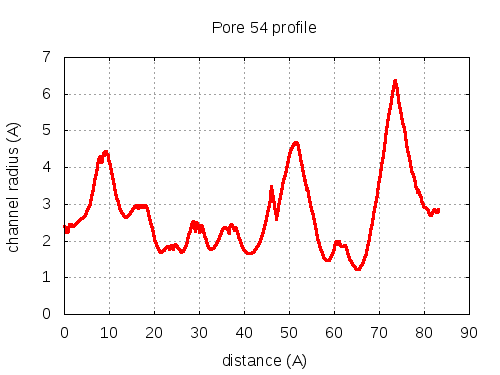
Unique lining residues set - all
124 HIS C, 200122 ARG C, 200124 HIS C, 200119 GLN C, 124 HIS C, 119 GLN C, 100119 GLN C, 100124 HIS C, 300119 GLN C, 300124 HIS C, 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 104 GLY C, 100 ILE C, 100100 ILE C, 200100 ILE C, 300100 ILE C, 100104 GLY C, 100103 PHE C, 300103 PHE C, 100101 THR C, 100100 ILE C, 300106 VAL C, 300110 LEU C, 100101 THR C
Unique lining residues set - sidechains
200122 ARG C, 200124 HIS C, 200119 GLN C, 124 HIS C, 119 GLN C, 100119 GLN C, 100124 HIS C, 300119 GLN C, 300124 HIS C, 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100 ILE C, 100100 ILE C, 200100 ILE C, 300100 ILE C, 100103 PHE C, 300106 VAL C, 300110 LEU C, 100101 THR C
Physicochemical properties of lining side-chains
Charge: 1 (1-0)
Hydropathy: 0.4
Hydrophobicity: -0.01
Polarity: 6.13
Mutability: 93
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 124 HIS C, 200122 ARG C, 200124 HIS C 2.25 0.83 -2.7 -0.32 35.66 87 2 124 HIS C, 200122 ARG C, 200124 HIS C, 200119 GLN C 2.25 1.89 -2.9 -0.52 27.63 86 3 200124 HIS C, 200119 GLN C, 124 HIS C 2.37 5.63 -3.3 -0.19 35.58 88 4 200124 HIS C, 200119 GLN C, 124 HIS C, 119 GLN C 3 7.4 -3.35 -0.42 27.57 87 5 200124 HIS C, 200119 GLN C, 124 HIS C, 119 GLN C, 100119 GLN C 4.04 7.97 -3.38 -0.56 22.76 86 6 119 GLN C, 100119 GLN C, 100124 HIS C, 300119 GLN C, 300124 HIS C 4.16 8.72 -3.38 -0.56 22.76 86 7 200124 HIS C, 200119 GLN C, 124 HIS C, 119 GLN C, 100119 GLN C 4.33 8.97 -3.38 -0.56 22.76 86 8 200124 HIS C, 124 HIS C, 100124 HIS C, 300119 GLN C, 300124 HIS C 4.4 9.43 -3.26 -0.01 41.99 89 9 200119 GLN C, 124 HIS C, 119 GLN C, 100119 GLN C, 300119 GLN C 3.54 11.64 -3.44 -0.83 13.14 85 10 200119 GLN C, 119 GLN C, 100119 GLN C, 300119 GLN C 2.67 16.92 -3.5 -1.1 3.53 84 11 200119 GLN C, 119 GLN C, 100119 GLN C, 300119 GLN C, 200115 VAL C 3 17.01 -1.96 -0.65 2.85 86 12 300119 GLN C, 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C 2.9 17.38 2.66 0.68 0.81 95 13 200119 GLN C, 119 GLN C, 100119 GLN C, 300119 GLN C 2.91 18.62 -3.5 -1.1 3.53 84 14 119 GLN C, 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C 2.2 20.54 2.66 0.68 0.81 95 15 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C 1.7 27.63 4.2 1.13 0.13 98 16 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C, 100111 ALA C 2.19 28.72 3.72 0.91 0.1 98 17 115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.27 28.91 2.28 0.24 0.03 99 18 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.27 29.65 2.28 0.24 0.03 99 19 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C, 111 ALA C 2.39 29.9 3.72 0.91 0.1 98 20 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C, 300111 ALA C 2.2 31.44 3.72 0.91 0.1 98 21 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.15 32.54 2.28 0.24 0.03 99 22 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 1.77 34.87 1.8 0.02 0 100 23 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C 2.15 36.27 1.3 -0.14 0.33 101 24 300111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 2.21 37.15 -0.2 -0.61 1.33 105 25 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C 2.24 38.8 1.3 -0.14 0.33 101 26 100111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 2.18 39.92 -0.2 -0.61 1.33 105 27 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 1.6 49.65 -0.7 -0.77 1.66 107 28 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 104 GLY C 4.25 50.28 -0.64 -0.78 2 107 29 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100 ILE C 4.62 50.72 0.34 -0.25 1.35 106 30 100107 THR C, 100 ILE C, 100100 ILE C, 200100 ILE C, 300100 ILE C 4.62 51.27 3.46 1.29 0.44 103 31 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100 ILE C 4.7 51.32 0.34 -0.25 1.35 106 32 107 THR C, 200107 THR C, 300107 THR C, 100 ILE C, 300100 ILE C 4.65 51.55 1.38 0.26 1.05 105 33 100107 THR C, 200107 THR C, 300107 THR C, 100100 ILE C, 200100 ILE C 4.54 51.98 1.38 0.26 1.05 105 34 100107 THR C, 300107 THR C, 100100 ILE C, 200100 ILE C, 100104 GLY C 4.09 52.73 1.44 0.26 1.39 105 35 100107 THR C, 100100 ILE C, 200100 ILE C, 100104 GLY C 3.83 53.06 1.98 0.51 1.33 104 36 100100 ILE C, 200100 ILE C, 100104 GLY C, 100103 PHE C 3.57 53.44 2.85 1.04 1 85 37 100100 ILE C, 100104 GLY C, 100103 PHE C 3.22 54.36 2.3 0.79 1.29 77 38 300107 THR C, 100100 ILE C, 100104 GLY C 1.68 57.86 1.13 0.08 1.72 105 39 300107 THR C, 100100 ILE C, 100104 GLY C, 300103 PHE C 1.47 58.7 0.75 -0.14 2.14 105 40 300107 THR C, 100100 ILE C, 300103 PHE C 1.46 59.86 1.13 0.08 1.72 105 41 300107 THR C, 100100 ILE C, 300103 PHE C, 100101 THR C 1.85 60.62 0.75 -0.14 2.14 105 42 300107 THR C, 300103 PHE C, 100101 THR C, 100100 ILE C, 300106 VAL C 1.96 60.76 0.46 -0.41 2.39 102 43 300107 THR C, 300103 PHE C, 100101 THR C, 300106 VAL C 1.84 61.74 0.68 -0.31 2.14 102 44 300107 THR C, 100101 THR C, 300106 VAL C 1.83 62 1.03 -0.15 1.72 102 45 300107 THR C, 100101 THR C, 300106 VAL C, 300110 LEU C 1.77 62.65 1.73 0.18 1.33 86 46 300106 VAL C, 300110 LEU C, 100101 THR C 1.24 66.98 2.43 0.5 0.64 86 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
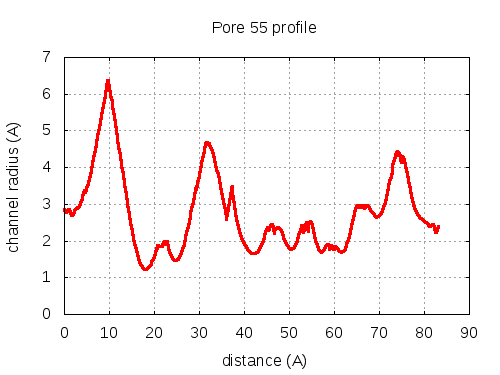
Unique lining residues set - all
100124 HIS C, 300122 ARG C, 300124 HIS C, 300119 GLN C, 100124 HIS C, 100119 GLN C, 119 GLN C, 124 HIS C, 200119 GLN C, 200124 HIS C, 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100104 GLY C, 100100 ILE C, 100 ILE C, 200100 ILE C, 300100 ILE C, 104 GLY C, 103 PHE C, 200103 PHE C, 101 THR C, 100 ILE C, 200106 VAL C, 200110 LEU C, 101 THR C
Unique lining residues set - sidechains
300122 ARG C, 300124 HIS C, 300119 GLN C, 100124 HIS C, 100119 GLN C, 119 GLN C, 124 HIS C, 200119 GLN C, 200124 HIS C, 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100100 ILE C, 100 ILE C, 200100 ILE C, 300100 ILE C, 103 PHE C, 200106 VAL C, 200110 LEU C, 101 THR C
Physicochemical properties of lining side-chains
Charge: 1 (1-0)
Hydropathy: 0.5
Hydrophobicity: 0.01
Polarity: 6
Mutability: 93
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 100124 HIS C, 300122 ARG C, 300124 HIS C 2.25 0.83 -2.7 -0.32 35.66 87 2 100124 HIS C, 300122 ARG C, 300124 HIS C, 300119 GLN C 2.25 1.89 -2.9 -0.52 27.63 86 3 300124 HIS C, 300119 GLN C, 100124 HIS C 2.37 5.63 -3.3 -0.19 35.58 88 4 300124 HIS C, 300119 GLN C, 100124 HIS C, 100119 GLN C 3 7.4 -3.35 -0.42 27.57 87 5 300124 HIS C, 300119 GLN C, 100124 HIS C, 100119 GLN C, 119 GLN C 4.04 7.97 -3.38 -0.56 22.76 86 6 100119 GLN C, 119 GLN C, 124 HIS C, 200119 GLN C, 200124 HIS C 4.16 8.72 -3.38 -0.56 22.76 86 7 300124 HIS C, 300119 GLN C, 100124 HIS C, 100119 GLN C, 119 GLN C 4.33 8.97 -3.38 -0.56 22.76 86 8 300124 HIS C, 100124 HIS C, 124 HIS C, 200119 GLN C, 200124 HIS C 4.4 9.43 -3.26 -0.01 41.99 89 9 300119 GLN C, 100124 HIS C, 100119 GLN C, 119 GLN C, 200119 GLN C 3.54 11.64 -3.44 -0.83 13.14 85 10 300119 GLN C, 100119 GLN C, 119 GLN C, 200119 GLN C 2.67 16.92 -3.5 -1.1 3.53 84 11 300119 GLN C, 100119 GLN C, 119 GLN C, 200119 GLN C, 200115 VAL C 3 17.01 -1.96 -0.65 2.85 86 12 119 GLN C, 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C 2.9 17.22 2.66 0.68 0.81 95 13 300119 GLN C, 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C 2.96 17.38 2.66 0.68 0.81 95 14 300119 GLN C, 100119 GLN C, 119 GLN C, 200119 GLN C 2.91 18.62 -3.5 -1.1 3.53 84 15 119 GLN C, 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C 2.2 20.54 2.66 0.68 0.81 95 16 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C 1.7 27.63 4.2 1.13 0.13 98 17 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C, 100111 ALA C 2.19 28.72 3.72 0.91 0.1 98 18 115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.27 28.91 2.28 0.24 0.03 99 19 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.27 29.65 2.28 0.24 0.03 99 20 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C, 111 ALA C 2.39 29.9 3.72 0.91 0.1 98 21 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C, 300111 ALA C 2.2 31.44 3.72 0.91 0.1 98 22 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.15 32.54 2.28 0.24 0.03 99 23 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 1.77 34.87 1.8 0.02 0 100 24 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C 2.15 36.27 1.3 -0.14 0.33 101 25 300111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 2.21 37.15 -0.2 -0.61 1.33 105 26 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300107 THR C 2.24 38.8 1.3 -0.14 0.33 101 27 100111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 2.18 39.92 -0.2 -0.61 1.33 105 28 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 1.6 49.65 -0.7 -0.77 1.66 107 29 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100104 GLY C 4.25 50.28 -0.64 -0.78 2 107 30 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100100 ILE C 4.62 50.72 0.34 -0.25 1.35 106 31 107 THR C, 100100 ILE C, 100 ILE C, 200100 ILE C, 300100 ILE C 4.62 51.27 3.46 1.29 0.44 103 32 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100100 ILE C 4.7 51.32 0.34 -0.25 1.35 106 33 100107 THR C, 200107 THR C, 300107 THR C, 100100 ILE C, 200100 ILE C 4.65 51.55 1.38 0.26 1.05 105 34 107 THR C, 200107 THR C, 300107 THR C, 100 ILE C, 300100 ILE C 4.54 51.98 1.38 0.26 1.05 105 35 107 THR C, 200107 THR C, 100 ILE C, 300100 ILE C, 104 GLY C 4.09 52.73 1.44 0.26 1.39 105 36 107 THR C, 100 ILE C, 300100 ILE C, 104 GLY C 3.83 53.06 1.98 0.51 1.33 104 37 100 ILE C, 300100 ILE C, 104 GLY C, 103 PHE C 3.57 53.44 2.85 1.04 1 85 38 100 ILE C, 104 GLY C, 103 PHE C 3.22 54.36 2.3 0.79 1.29 77 39 200107 THR C, 100 ILE C, 104 GLY C 1.68 57.86 1.13 0.08 1.72 105 40 200107 THR C, 100 ILE C, 104 GLY C, 200103 PHE C 1.47 58.7 0.75 -0.14 2.14 105 41 200107 THR C, 100 ILE C, 200103 PHE C 1.46 59.86 1.13 0.08 1.72 105 42 200107 THR C, 100 ILE C, 200103 PHE C, 101 THR C 1.85 60.62 0.75 -0.14 2.14 105 43 200107 THR C, 200103 PHE C, 101 THR C, 100 ILE C, 200106 VAL C 1.96 60.76 0.46 -0.41 2.39 102 44 200107 THR C, 200103 PHE C, 101 THR C, 200106 VAL C 1.84 61.74 0.68 -0.31 2.14 102 45 200107 THR C, 101 THR C, 200106 VAL C 1.83 62 1.03 -0.15 1.72 102 46 200107 THR C, 101 THR C, 200106 VAL C, 200110 LEU C 1.77 62.65 1.73 0.18 1.33 86 47 200106 VAL C, 200110 LEU C, 101 THR C 1.24 66.98 2.43 0.5 0.64 86 pore with bottle neck
pore with local minimum
-
show | | profile | lining residues
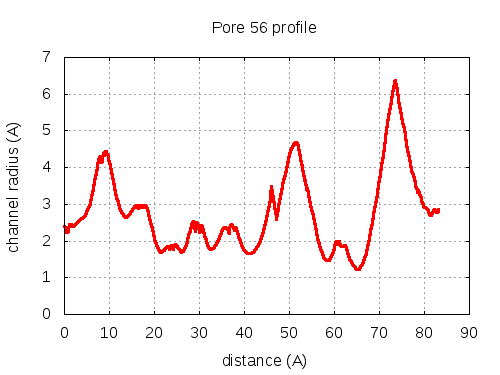
Unique lining residues set - all
124 HIS C, 200122 ARG C, 200124 HIS C, 200119 GLN C, 124 HIS C, 119 GLN C, 100119 GLN C, 100124 HIS C, 300119 GLN C, 300124 HIS C, 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 200104 GLY C, 100 ILE C, 100100 ILE C, 200100 ILE C, 300100 ILE C, 200103 PHE C, 100103 PHE C, 200101 THR C, 100106 VAL C, 200100 ILE C, 100110 LEU C, 200101 THR C
Unique lining residues set - sidechains
200122 ARG C, 200124 HIS C, 200119 GLN C, 124 HIS C, 119 GLN C, 100119 GLN C, 100124 HIS C, 300119 GLN C, 300124 HIS C, 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100 ILE C, 100100 ILE C, 200100 ILE C, 300100 ILE C, 200103 PHE C, 100106 VAL C, 100110 LEU C, 200101 THR C
Physicochemical properties of lining side-chains
Charge: 1 (1-0)
Hydropathy: 0.4
Hydrophobicity: -0.01
Polarity: 5.76
Mutability: 93
Lining residues
show all | hide all
# Res Btn Dist Hpa Hpb Pol Mut 1 124 HIS C, 200122 ARG C, 200124 HIS C 2.25 0.85 -2.7 -0.32 35.66 87 2 124 HIS C, 200122 ARG C, 200124 HIS C, 200119 GLN C 2.26 1.57 -2.9 -0.52 27.63 86 3 200124 HIS C, 200119 GLN C, 124 HIS C 2.38 5.92 -3.3 -0.19 35.58 88 4 200124 HIS C, 200119 GLN C, 124 HIS C, 119 GLN C 3.14 7.63 -3.35 -0.42 27.57 87 5 200124 HIS C, 200119 GLN C, 124 HIS C, 119 GLN C, 100119 GLN C 4.21 8.11 -3.38 -0.56 22.76 86 6 119 GLN C, 100119 GLN C, 100124 HIS C, 300119 GLN C, 300124 HIS C 4.15 8.75 -3.38 -0.56 22.76 86 7 200124 HIS C, 200119 GLN C, 124 HIS C, 119 GLN C, 100119 GLN C 4.33 9.01 -3.38 -0.56 22.76 86 8 200124 HIS C, 124 HIS C, 100124 HIS C, 300119 GLN C, 300124 HIS C 4.42 9.35 -3.26 -0.01 41.99 89 9 200119 GLN C, 124 HIS C, 119 GLN C, 100119 GLN C, 300119 GLN C 3.61 11.5 -3.44 -0.83 13.14 85 10 200119 GLN C, 119 GLN C, 100119 GLN C, 300119 GLN C 2.65 16.93 -3.5 -1.1 3.53 84 11 200119 GLN C, 119 GLN C, 100119 GLN C, 300119 GLN C, 200115 VAL C 2.98 17.02 -1.96 -0.65 2.85 86 12 300119 GLN C, 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C 2.9 17.27 2.66 0.68 0.81 95 13 200119 GLN C, 119 GLN C, 100119 GLN C, 300119 GLN C, 300115 VAL C 3.01 17.47 -1.96 -0.65 2.85 86 14 200119 GLN C, 119 GLN C, 100119 GLN C, 300119 GLN C 2.92 18.34 -3.5 -1.1 3.53 84 15 119 GLN C, 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C 2.41 20.17 2.66 0.68 0.81 95 16 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C 1.7 27.66 4.2 1.13 0.13 98 17 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C, 100111 ALA C 2.2 28.75 3.72 0.91 0.1 98 18 115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.27 28.95 2.28 0.24 0.03 99 19 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.29 29.43 2.28 0.24 0.03 99 20 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C, 111 ALA C 2.36 29.97 3.72 0.91 0.1 98 21 200115 VAL C, 115 VAL C, 100115 VAL C, 300115 VAL C, 300111 ALA C 2.21 30.82 3.72 0.91 0.1 98 22 300115 VAL C, 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 2.44 31.79 2.28 0.24 0.03 99 23 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C 1.72 35.28 1.8 0.02 0 100 24 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 107 THR C 2.35 36.03 1.3 -0.14 0.33 101 25 300111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 2.2 37.36 -0.2 -0.61 1.33 105 26 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 300107 THR C 2.2 38 1.3 -0.14 0.33 101 27 100111 ALA C, 111 ALA C, 200111 ALA C, 300111 ALA C, 100107 THR C 2.43 39.49 1.3 -0.14 0.33 101 28 100111 ALA C, 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 2.03 40.87 -0.2 -0.61 1.33 105 29 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C 1.54 49.7 -0.7 -0.77 1.66 107 30 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 200104 GLY C 4.24 50.33 -0.64 -0.78 2 107 31 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100 ILE C 4.53 50.74 0.34 -0.25 1.35 106 32 300107 THR C, 100 ILE C, 100100 ILE C, 200100 ILE C, 300100 ILE C 4.65 51.28 3.46 1.29 0.44 103 33 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 100 ILE C 4.7 51.32 0.34 -0.25 1.35 106 34 107 THR C, 100107 THR C, 200107 THR C, 300107 THR C, 200100 ILE C 4.69 51.41 0.34 -0.25 1.35 106 35 107 THR C, 100107 THR C, 200107 THR C, 100 ILE C, 200100 ILE C 4.69 51.46 1.38 0.26 1.05 105 36 107 THR C, 300107 THR C, 100 ILE C, 100100 ILE C, 300100 ILE C 4.62 51.81 2.42 0.78 0.74 104 37 107 THR C, 100107 THR C, 200107 THR C, 100 ILE C, 200100 ILE C 4.61 52.03 1.38 0.26 1.05 105 38 100107 THR C, 200107 THR C, 200104 GLY C, 100 ILE C, 200100 ILE C 4 53.01 1.44 0.26 1.39 105 39 200107 THR C, 200104 GLY C, 100 ILE C, 200100 ILE C 3.86 53.17 1.98 0.51 1.33 104 40 200104 GLY C, 100 ILE C, 200100 ILE C, 200103 PHE C 3.58 53.58 2.85 1.04 1 85 41 200104 GLY C, 200100 ILE C, 200103 PHE C 3.19 54.59 2.3 0.79 1.29 77 42 100107 THR C, 200104 GLY C, 200100 ILE C 1.6 58.31 1.13 0.08 1.72 105 43 100107 THR C, 200100 ILE C, 100103 PHE C 1.43 60.13 1.13 0.08 1.72 105 44 100107 THR C, 200100 ILE C, 100103 PHE C, 200101 THR C 1.9 60.7 0.75 -0.14 2.14 105 45 100107 THR C, 100103 PHE C, 200101 THR C, 100106 VAL C, 200100 ILE C 1.95 61.01 0.46 -0.41 2.39 102 46 100107 THR C, 100103 PHE C, 200101 THR C, 100106 VAL C 1.86 61.79 0.68 -0.31 2.14 102 47 100107 THR C, 200101 THR C, 100106 VAL C 1.83 62.08 1.03 -0.15 1.72 102 48 100107 THR C, 200101 THR C, 100106 VAL C, 100110 LEU C 1.79 62.76 1.73 0.18 1.33 86 49 100106 VAL C, 100110 LEU C, 200101 THR C 1.24 67.12 2.43 0.5 0.64 86 pore with bottle neck
pore with local minimum
|
34 cavities click to expand / contract
- (volume ~ 54050 Å3)
- (volume ~ 5878 Å3)
- (volume ~ 871 Å3)
- (volume ~ 871 Å3)
- (volume ~ 871 Å3)
- (volume ~ 871 Å3)
- (volume ~ 819 Å3)
- (volume ~ 819 Å3)
- (volume ~ 819 Å3)
- (volume ~ 819 Å3)
- (volume ~ 608 Å3)
- (volume ~ 608 Å3)
- (volume ~ 608 Å3)
- (volume ~ 608 Å3)
- (volume ~ 318 Å3)
- (volume ~ 318 Å3)
- (volume ~ 318 Å3)
- (volume ~ 318 Å3)
- (volume ~ 193 Å3)
- (volume ~ 193 Å3)
- (volume ~ 193 Å3)
- (volume ~ 193 Å3)
- (volume ~ 192 Å3)
- (volume ~ 192 Å3)
- (volume ~ 192 Å3)
- (volume ~ 192 Å3)
- (volume ~ 190 Å3)
- (volume ~ 190 Å3)
- (volume ~ 190 Å3)
- (volume ~ 190 Å3)
- (volume ~ 92 Å3)
- (volume ~ 92 Å3)
- (volume ~ 92 Å3)
- (volume ~ 92 Å3)